Epidural bupivacaine administration after infiltration of liposomal bupivacaine for transversalis fascia plane block: A case report
Abstract
Extended-release liposomal bupivacaine is frequently used in surgical infiltration for postoperative pain control. The manufacturer recommends against subsequent local anesthetics within 96 hours. We administered epidural bupivacaine one day after local liposomal bupivacaine infiltration for staged hemipelvectomy without symptoms of LAST. Further pharmacokinetic and clinical safety studies are needed.
1 INTRODUCTION
Liposomal bupivacaine, an extended-release local anesthetic, is frequently used in surgical infiltration and fascial plane blocks to improve postoperative pain control and minimize narcotics.1 It has also been approved for use in interscalene brachial plexus blocks, but not other peripheral nerve blocks. To the best of our knowledge, there are no reports of local anesthetic administration after liposomal bupivacaine administration. We present a unique case in which bupivacaine was administered epidurally for postoperative pain control one day after receiving liposomal bupivacaine via a transversalis fascia plane (TFP) block during a staged hemipelvectomy.
2 CASE REPORT
The patient approved the reporting of this case and written Health Insurance Portability and Accountability Act (HIPAA) authorization and informed consent for publication was obtained in preparation.
A 72-year-old male patient with a history of hypertension and hyperlipidemia presented for a hemipelvectomy and hemisacrectomy with soft tissue reconstruction for an incidentally found pelvic chondrosarcoma. Due to the complex anatomy surrounding the tumor, a staged approach was pursued to free and preserve the surrounding structures in the surgical field. The first stage consisted of a laparoscopic low anterior mobilization of the rectum and cecum with ureteral stent placement. Preoperatively, the patient received 1000 mg oral acetaminophen and 10 mg extended release oxycodone. Intraoperatively, the patient received 150 mcg intravenous fentanyl and 50 mg intravenous ketamine for analgesia. The procedure was uncomplicated and concluded with bilateral laparoscopic TFP blocks performed by the surgeon, using a mixture of 30 ml of 0.25% bupivacaine and 20 ml (266 mg) of liposomal bupivacaine. He did not require opioids while in the post anesthesia care unit (PACU) and only received 15 mg intravenous ketorolac. Later that evening, he required 5 mg oral oxycodone and an additional 15 mg intravenous ketorolac while receiving scheduled 1000 mg oral acetaminophen every 6 h.
The following day, the patient underwent the second stage which consisted of the internal hemipelvectomy and hemisacrectomy. In anticipation of uncontrolled postoperative pain with conventional oral and intravenous analgesics, we considered an epidural with combined local anesthetic and opioid solution despite the recent administration of liposomal bupivacaine. In our clinical experience, patients undergoing hemipelvectomy procedures often require epidural analgesia for improved pain control in the postoperative period. In this case, we were aware of the unconventional nature of this decision, therefore, we discussed postoperative pain control options with the patient, including epidural analgesia. The risks, benefits, and alternatives were discussed with the patient including the particular risk of local anesthetic systemic toxicity (LAST) and the patient ultimately decided to pursue epidural analgesia given the extent of the surgery and anticipated postoperative pain. Preoperatively, the patient received 75 mcg intravenous fentanyl during an uncomplicated lumbar epidural placement. Intraoperatively, the patient received 225 mcg intravenous fentanyl and 50 mg intravenous ketamine, as well as a dexmedetomidine infusion at 0.3–0.4 mcg/kg/h. Prior to extubation, the patient was given a total of 15 ml of 0.125% bupivacaine epidurally in divided doses.
On arrival to PACU, an epidural infusion of 0.1% bupivacaine and 2 mcg/ml fentanyl was started with the following parameters: basal rate 6 ml/h, patient-controlled bolus dose 2 ml/dose with a 15-min lockout, maximum dose 18 ml/h. In PACU, he did not require additional opioids or on-demand epidural doses. The patient was discharged from PACU to an intermediate care unit. During his hospitalization, the patient received scheduled 400 mg oral ibuprofen every 6 h and 1000 mg oral acetaminophen every 6 h. He received three epidural demand doses on postoperative day (POD) 0. The following morning the patient complained of dizziness which was attributed to the fentanyl component of his epidural solution. These symptoms resolved after removal of the fentanyl from the epidural solution. On POD 1 and 2, the patient did not require opioid analgesics or epidural demand doses while receiving epidural bupivacaine. During this timeframe, his numerical rating pain scores ranged from 0 to 2 out of a maximum of 10 (with 10 being the worst imaginable pain). The epidural was removed on POD 2 and 5–10 mg oxycodone every 4 h as needed was added to his multimodal regimen. The patient did not exhibit signs of LAST during his hospitalization. His overall opioid requirements were titrated over the following days, and the patient was discharged on POD 8 to an acute rehabilitation center.
3 DISCUSSION
Postoperative pain management can be challenging, especially in extensive staged resections. Adequate postoperative pain control is an important consideration in a patient's ability to achieve optimal recovery. The colorectal surgical team conducting the first stage performed a TFP block using liposomal bupivacaine as is typical in their practice. While this provided excellent pain control after bowel mobilization, the use of liposomal bupivacaine complicated postoperative pain control after the hemipelvectomy, which commonly necessitates epidural analgesia in our clinical practice. Ideally, all members of the team, including the patient, should be involved in planning the postoperative pain control regimen prior to the staged procedure, particularly when liposomal bupivacaine administration is anticipated.
Local anesthetic systemic toxicity is a potential concern in patients receiving local anesthetic after liposomal bupivacaine, especially in the first 96 h, due to the delicate delivery system of liposomal bupivacaine. LAST can present with both central nervous system and cardiovascular symptoms.2 These can vary from metallic taste, tinnitus, and peri-oral numbness to bradycardia or tachycardia, and potentially fatal arrhythmias. Compared with other local anesthetics, bupivacaine possesses cardiotoxic properties that decrease pacemaker activity and prolongs the refractory period of cardiac myocytes.3 Maximum dosing has been established for single-agent local anesthetic uses. When combining different local anesthetics, dosing is complicated as the negative effects are additive.4 The use of extended-release formulations complicates dosing as standards have not been established when used with other local anesthetics.
After extensive discussion of the risks, benefits, and alternatives amongst the surgeons, anesthesiologist, and patient, an epidural with bupivacaine infusion was chosen as part of the postoperative pain regimen. As a result, we were able to successfully employ an adequate multimodal opioid-sparing analgesic regimen without evidence of LAST. Postoperatively, the patient was discharged from the PACU to an intermediate care unit with increased nursing availability with a ratio of one nurse to two patients allowing for more frequent monitoring and rounding of postoperative patients. Additionally, the nursing staff on this unit is familiar with the use of epidurals in orthopedic surgical patients. The increasing nursing availability added a level of safety regarding the ability to detect signs of LAST more rapidly and notify providers for prompt evaluation.
Liposomal bupivacaine employs a patented delivery mechanism consisting of multivesicular liposomes (MVL) which are comprised of various drug-containing chambers separated by lipid membranes. When locally infiltrated, the MVLs release bupivacaine over a maximum period of 96 hours.5 As seen in pharmacokinetic studies (Figure 1), there is an initial peak in plasma bupivacaine concentration after infiltration of liposomal bupivacaine that dramatically decreases within the first few hours. This peak is attributed to the approximately 3% of free bupivacaine molecules within the vials along with the initial release from the exterior vesicles.6, 7 The vesicles undergo rearrangement to prevent additional rapid release of drug allowing for a more controlled secondary release of drug through erosion of lipid membranes when in contact with the surrounding tissue layers. There is a second peak observed approximately 12 h after infiltration with Food and Drug Administration (FDA) approved doses of 106 mg and 266 mg. After the second peak, concentrations are sustained due to the delivery system with a steady decline until 96 h after administration. The liposomal structure can be disrupted, releasing excess drug by various factors such as temperature change, needle gauge, and diluent compatibility. As a result, the manufacturer recommends storing the vials under refrigeration and utilizing a 25 gauge or larger bore needle.8 The manufacturer warns of a risk of immediate release of bupivacaine from the liposomes if administered with other non-bupivacaine local anesthetics. Additionally, there is a recommendation to avoid the use of additional local anesthetics within 96 h after administration of liposomal bupivacaine as plasma concentrations may persist within this timeframe potentiating a risk of LAST.8
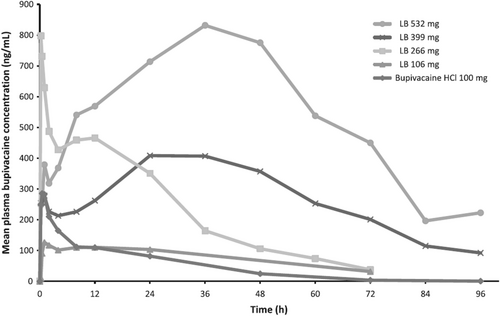
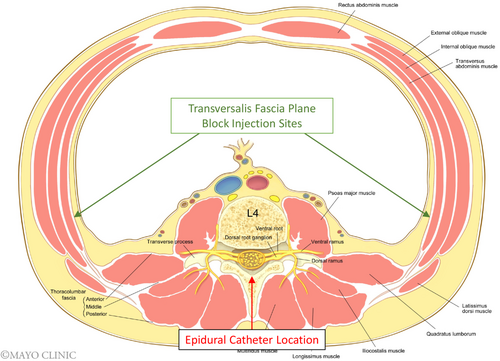
Based on the pharmacokinetic profile of liposomal bupivacaine, the risk of toxicity is likely greatest within the first 24 h given that both peaks occur in this timeframe. In our case, greater than 24 h passed between liposomal bupivacaine infiltration and the first epidural bolus dose. We hypothesized that the risk of LAST with epidural bupivacaine administration in our patient, was theoretically lower as the drug would not be infusing into the TFP, thus, decreasing the chance of directly interacting with the liposomal particles (Figure 2). Bupivacaine was purposefully chosen for the epidural solution given the potential for immediate release of bupivacaine from the liposomes if mixed with a non-bupivacaine local anesthetic. In our case, we hypothesized that the highest risk timeframe for LAST would have been between 24 and 36 h given the pharmacokinetic profile of liposomal bupivacaine and the concurrent use of epidural bupivacaine during this period. However, toxicity remained a concern up to 96 hours post-infiltration due to the potential for persistent plasma levels of bupivacaine. The only adverse effect reported by the patient was dizziness, which could be multifactorial including the residual effects of anesthesia, volume depletion, and neuraxial opioid. Given the resolution of the dizziness after removal of fentanyl from the epidural solution, we believe it was the most likely cause for the patient's symptoms.
In summary, we present a case in which epidural bupivacaine was administered one day after liposomal bupivacaine administration in a TFP block. Although our patient received local anesthetic safely within 96 h of liposomal bupivacaine administration, we are not advocating for the routine use of local anesthetic after liposomal bupivacaine administration. Further pharmacokinetic and clinical safety studies are needed to evaluate the potential toxic plasma concentrations of bupivacaine when local anesthetic is administered after liposomal bupivacaine administration. These studies may guide safe and expanded use of local anesthetics as we continue to aim for opioid-sparing multimodal perioperative pain management.
AUTHOR CONTRIBUTIONS
Monica Harbell helped with data acquisition, manuscript writing, editing, and final approval of manuscript. William Binder helped with data acquisition, manuscript writing, editing, and final approval of manuscript. Colby Erickson helped with data acquisition, manuscript writing, editing, and final approval of manuscript. Krista Goulding helped with data acquisition, editing, and final approval of manuscript. Molly Kraus helped with data acquisition, manuscript writing, editing, and final approval of manuscript.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
Dr. Harbell was an educational consultant for the American Society of Regional Anesthesia and Pain Medicine ending in 2020. The remaining authors report no conflicts of interest.
ETHICal APPROVAL
As the case report is devoid of patient identifiable information, it is exempt from IRB review requirements as per Mayo Clinic policy.
CONSENT
The patient approved the reporting of this case and written Health Insurance Portability and Accountability Act (HIPAA) authorization and informed consent for publication was obtained in preparation.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during this case report.