Psychogenic polydipsia in a female adolescent without a psychiatric background: A case report
Abstract
Psychological stress is a risk factor for primary polydipsia in adolescents without psychiatric comorbidity. Taking a detailed family and social history can help to distinguish primary polydipsia from diabetes insipidus in adolescents with challenging presentations of polydipsia and polyuria.
1 INTRODUCTION
Psychological stress is a risk factor for primary polydipsia in adolescents without psychiatric comorbidity. Taking a detailed family and social history can help to distinguish primary polydipsia from diabetes insipidus in adolescents with challenging presentations of polydipsia and polyuria.
Polydipsia in children is defined as water volume consumption exceeding 2 L/m2/day.1, 2 Polydipsia is caused by several conditions including dehydration, exaggerated exercise, excessive water drinking (primary polydipsia), hyperglycemia (diabetes mellitus), or diabetes insipidus (DI) (central or nephrogenic). Primary polydipsia (PP) is a clinical disorder characterized by excessive thirst and a pathologically high fluid intake leading to hypotonic polyuria and a decreased serum osmolality.
Four types of PP can be distinguished: (a) polydipsia in patients with neurodevelopmental disorders (such as autism and intellectual disabilities) and chronic psychotic disorders (such as schizophrenia, schizoaffective disorder, bipolar disorder, and psychotic depression), (b) psychogenic polydipsia or compulsive water drinking, that is, polydipsia caused by psychiatric conditions such as obsessive-compulsive disorder, alcoholism, anxiety disorder, depression, and anorexia nervosa, (c) dipsogenic polydipsia, that is, polydipsia in patients with excessive thirst due to hypothalamic lesions caused by trauma, vascular or infiltrative diseases or patients with habitual polydipsia, and (d) iatrogenic polydipsia, that is, medical advice or act that leads to increased fluid intake.3-8 During adolescence, PP has mainly been reported in young women with psychiatric comorbidity (Table 1).9-12 Here, we report the sudden onset of excessive drinking of mainly cold water as an atypical presentation of PP in a 14-year-old girl, as a coping strategy for acute family stress due to parental burnout.
| Case report | Patient age | Complaints | Diagnosis | Reference |
|---|---|---|---|---|
| 1 |
16 y |
- 10-11 L/d for 6 mo - Gradual onset |
Obsessive-Compulsive Disorder | 9 |
| 12 y | 10-12 L for 7 mo | Obsessive-Compulsive Disorder | ||
| 2 | 13 y | 4 L/d for weeks | Anorexia Nervosa | 10 |
| 3 | 18 y | 2.5 L/2 h | Mild mental retardation (+ use of diuretics) | 11 |
| 14 y | unspecified duration and onset |
Attention Deficit Hyperactivity Disorder |
||
| 4 | 18 y | 8 L/d during years | Anorexia Nervosa | 12 |
| 16 y | 6-7 L/d, unspecified duration | Anorexia Nervosa |
2 CASE REPORT/CASE PRESENTATION
A 14-year-old girl with complaints of acute polydipsia and polyuria in the last 5 days and a normal plasma glucose and negative urine dipstick analysis for glucose and protein was referred for suspicion of DI. She had a sudden onset of abnormal craving for cold fluids. Her fluid intake was ca. 10 liters of mostly cold water per day and she passed large volumes of urine during both day and night time. She had no fever, abdominal pain, vomiting, diarrhea, headache, or any other neurological changes. Her appetite remained constant and no weight changes occurred in the previous months. She had consulted her general practitioner for the same symptoms 2 days before presentation. Blood and urinary glucose levels and serum electrolytes were normal, and no glucosuria or proteinuria was detected by a dipstick urinalysis.
Her past medical history was unremarkable, and she was currently not taking any prescribed or over-the-counter medication. There was no family history of renal, endocrinological, or psychiatric diseases.
On examination, the mental and hydration status were normal. The blood pressure was 105/76 mm Hg, and the pulse 70 beats per minute; the temperature was normal at 37.2°C. The height was 154 cm (percentile 25), the weight 52.3 kg (percentile 50), and the body mass index (BMI; the weight in kilograms divided by the square of the height in meters) 22.0 kg/m2 (P75). Cranial nerve examination, muscle tonus, and deep tendon reflexes were normal. Her laboratory results showed a low serum and urine sodium and a low urine and serum osmolality (Table 2). Basal hormonal investigations, including adrenocorticotropic hormone (ACTH), thyroid-stimulating hormone (TSH), thyroxine, insulin-like growth factor (IGF), cortisol, and prolactin, were normal. Brain MRI (normal posterior pituitary and pituitary stalk) and kidney ultrasonography were normal.
| Variable | Result | Reference value |
|---|---|---|
| Serum | ||
| Sodium | 133 mmol/L | 135-145 mmol/L |
| Potassium | 3.6 mmol/L | 3.4-5 mmol/L |
| Chloride | 97 mmol/L | 96-109 mmol/L |
| Calcium | 2.42 mmol/L | 2.29-2.66mmol/L |
| Creatinine | 0.6 mg/dL | <1.1/dL |
| Osmolality | 261 mOsm/kg | 275-295 mOsm/kg |
| Urine | ||
| Creatinine | 10 mg/dL | |
| Sodium | 12 mmol/L | 20-40 mmol/L |
| Potassium | <2.5 mmol/L | 15-40mmol/L |
| Osmolality | 47 mOsm/kg | 500-800 mOsm/kg |
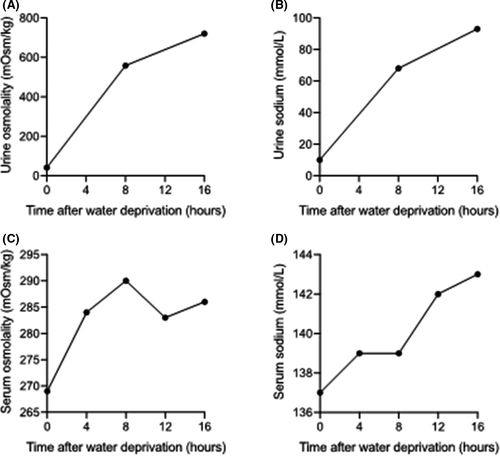
A 16-hour water deprivation test showed a rapid increase of urine osmolality up to 720mOsmol/kg, while serum sodium and osmolality remained within normal limits. These results were suggestive for PP (Figure 1). Taking the patient's social history revealed that her mother was experiencing a burnout that posed major psychological stress on her children and husband. Psychological counseling and support were provided with good result as one week later, daily water intake had decreased to 3.5 liters and urine production returned to normal.
3 DISCUSSION/CONCLUSION
Primary polydipsia in children is mostly seen in infancy or early childhood, when polydipsia becomes a habit after a period of increased thirst, for example, during a period of fever or hot weather.4, 5, 8, 13-15 Such habitual polydipsia has been shown to reset the thirst threshold resulting in prolonged excessive fluid intake.6, 16 In the rare cases of PP reported in adolescence, overt psychiatric comorbidity such as obsessive-compulsive disorder or anorexia nervosa was usually present.9-12 The pathogenesis of PP remains incompletely understood. One of the brain structures involved in its pathophysiology is the anterior hippocampal formation, which normally constrains the hypothalamic-pituitary-adrenal axis (HPAA) and antidiuretic hormone (ADH) response to psychological stressors via a relay that terminates in the paraventricular nucleus. Some hypothesize that the anterior hippocampal volume is decreased in patients with schizophrenia or depression, which leads to dysfunction of this AH projection, enhanced HPAA activity and an abnormal increase in ADH levels (causing increased thirst) and decrease in CNS oxytocin (causing the impaired social behaviors) during psychological stress.3, 17-19 Others think that the use of antidepressants and their anticholinergic side effects are the main culprits leading to excessive thirst.6 Other patients with PP, without psychotic disorders, are more likely to complain of excessive thirst as it is the product of psychological or cognitive influences on their thirst intensity. In patients with anorexia nervosa, excessive drinking is sometimes used as a coping strategy or to decrease hunger.3, 16 In patients with organic brain disorders, it occurs in conjunction with polyphagia.3 This patient had no known psychiatric comorbidity, but experienced the parental burnout as a very stressful event. Stress-induced increases in dopamine levels may indeed elicit PP in acute psychotic patients. Dopamine administration in animal models has been reported to initiate increased drinking.20 One might speculate that in this patient, acute stress resulted in increased dopamine signaling triggering the PP.
This case illustrates that the diagnosis of PP can be challenging. Laboratory data are often insufficient to differentiate PP from diabetes insipidus, especially central diabetes insipidus (CDI), due to a physiological suppression of serum antidiuretic hormone levels and a decreased renal concentrating ability in both conditions.5, 8, 13 Several clinical findings can aid in the differentiation: a sudden onset of polydipsia, excessive polyuria, preference for cold water, drinking at night, nocturia, and persistence of symptoms are suggestive of diabetes insipidus. However, clinical findings can be misleading as shown in a recent prospective study wherein more than 60% of patients with PP reported nightly drinking, most of them preferred cold beverages, and nearly 80% indicated a sustained character of symptoms.21, 22 In this patient, the age, the absence of a psychiatric background, the diuresis of 10 liters over 5 days, and the preference for cold water hinted at a diagnosis of CDI. This case report thus illustrates that clinical signs and symptoms are not specific or sensitive enough to differentiate between the different causes of the polyuria polydipsia syndrome.21, 22
This case also underscores the importance of differentiating between PP and CDI as a wrong diagnosis and treatment can cause harm to patients. This patient underwent a water restriction test, the gold standard to differentiate PP from CDI,4, 5 but it is important to note that this test provides only indirect data. Decreased ADH secretion or action is indirectly diagnosed through measurements of urine osmolality, that is, the renal concentrating ability, during a prolonged dehydration period, and through measurements of the percental increment in urinary osmolality after the administration of exogenous vasopressin (desmopressin) (Table 3). It is preferably executed in a hospital setting under close medical supervision because it is in general difficult to perform this correctly in adolescents or safely in younger children on an outpatient basis.5, 6, 10, 23 Unfortunately, this test cannot always differentiate PP from a partial form of diabetes insipidus as chronic polyuria in PP patients will reduce their maximal urinary concentration capacity, leading to an insufficient increase in urine osmolality during water deprivation, while on the other hand, diabetes insipidus (DI) patients may develop an increased renal sensitivity to circulating arginine vasopressin, causing a small but significant rise in urine osmolality during water deprivation, explaining its overall diagnostic accuracy of only 70%.21, 22, 24 The clearly increased urine osmolality observed after acute water restriction in our case, despite the ingestion of huge water volumes, is probably explained by the short interval between the onset of polyuria and the testing.22, 25 The complementary information of psychological stress as initiating event for the polydipsia as well as the quick normalization of fluid intake and urine osmolality after start of psychological counseling confirmed the suggested diagnosis of PP by water deprivation test in this case.
| Primary polydipsia | CDI | NDI | |||
|---|---|---|---|---|---|
| Before water deprivation test | Serum Na | <135 mmol/L | Normal or > 145 mmol/L | ||
| Serum osm | <280 mOsm/kg | Normal or > 300 mOsm/kg | |||
| Urine osm | <300 mOsm/kg | <300 mOsm/kg | |||
| After water deprivation test | Serum Na | Risk for hypoNa | Risk for hyperNa | ||
| Serum osm | 288-291 mOsm/L | >300 mOsm/L | |||
| Urine osm | 300-800 mOsm/L |
Complete CDI |
Complete NDI |
Partial CDI/NDI |
|
| <300 mOsm/L |
300-800 mOsm/L |
||||
|
After desmopressin |
Serum osm | No effect | Decrease | No effect | |
| Urine osm |
Increase with < 9% |
>2x original level or > 50% increase |
Increase with < 10% |
Increase with 9%-50% (partial NDI lesser than partial CDI) |
|
- Abbreviations: CDI, central diabetes insipidus; Na, Natrium; NDI, nephrogenic diabetes insipidus; Osm, osmolality.
To overcome the limitations of the water restriction test, the direct measurement of ADH upon osmotic stimulation was introduced. Recent research showed that the test could not reliably differentiate primary polydipsia from CDI, especially the partial form. This is possibly due to the lack of a precise definition of the normal physiological relationship between ADH and osmolarity.22 A second important limitation is that its diagnostic performance strongly depends on the serum osmolality level reached at the end of the stimulation test. Levels below 290mOsm/kg make it impossible to differentiate PP from CDI as all corresponding plasma ADH levels remain within the reference range.26 Because of its technical difficulties for correct collection and measurements, its limited availability in commercial laboratories and its limited gain in diagnostic accuracy ADH measurement during water deprivation, it is not frequently used in clinical practice.21, 24
A promising alternative is the measurement of copeptin, the C-terminal segment of the ADH precursor peptide. Copeptin has the same stimuli as ADH, that is, hyperosmolality and hypovolemia and its serum levels are similar to those of ADH.24 Copeptin is a sensitive and stable marker that is easily measured with sandwich immunoassays 24, 27 and, as opposed to ADH, only a small sample volume is required for its determination, no extraction steps or other preanalytical procedures are needed, and results can be available after just 0.5-2.5 hours.22 High baseline copeptin levels above 21.4pmol/L without prior thirsting indicate nephrogenic diabetes insipidus. When copeptin is < 21.4pmol/L, further differentiation is necessary by osmotic stimulation: Patients with CDI will have a normal to low level of copeptin < 4.9pmol/L while patients with primary polydipsia will show a rise in copeptin. It has a high diagnostic accuracy of 95%.21, 24 A disadvantage of copeptin measurements after osmotic stimulation is the need for plasma sodium levels to exceed 147mmol/L to obtain reliable results, which implies a higher need for close clinical and biochemical monitoring of the patient during the test as compared to a water deprivation test. Moreover, this test is contraindicated in patients with epilepsy or heart failure.21, 24, 27 The dangers inherent to the osmotic stimulation have prompted the use of arginine-mediated stimulation of, copeptin secretion as a simpler and safer alternative. Arginine is a nonosmotic stimulator of the anterior and posterior pituitary. When copeptin is measured 60 minutes after stimulation, a diagnostic accuracy of 94% is obtained with a cutoff value of 3.5 pmol/L.27 Such diagnostic accuracy is high for the challenging and clinically relevant discrimination between PP and partial DI. The improved feasibility and safety at a similar diagnostic accuracy as hypertonic saline stimulation make arginine-stimulated copeptin measurements a preferred diagnostic approach.27
The main risk of excessive drinking is the development of hyponatremia, which occurs when free fluid intake exceeds free fluid excretion. It is associated with increased rehospitalization rates, morbidity, and mortality.28 The occurrence is influenced by several risk factors impairing urine dilution capacity, examples are medication (due to the stimulation of ADH release, increasing sensitivity of kidneys to ADH, or increasing sensation of thirst), physical or psychological stress (due to the nonosmotic stimulation of ADH that is known as a stress hormone), acute consumption of large quantities of fluids, and low solute intake (due to decreasing kidneys excretory capacity of water).6, 29 If hyponatremia is not properly or acutely treated, there is a risk of serious complications such as brain edema, seizures, falls, and fractures as well as rhabdomyolysis and central pontine myelinolysis.3, 6, 30 A second possible complication is the decrease of the renal concentration capacity caused by a washout mechanism and downregulation of aquaporin 2 water channels. Other less frequent risks are bladder dilatation, hydronephrosis, renal failure, congestive heart failure due to the polyuria, and circulatory volume excess.6, 7, 31
Unfortunately, the treatment options for PP are scarce. The perfect therapy would be a voluntary reduction of water intake, but this is often not attainable as patients will not comply with this strategy. Behavioral therapy that includes education on the disease, relaxation therapy using biofeedback, and conditioning of desired behavior is a valid alternative. However, its implementation in clinical practice often proves difficult due to limited time and manpower. Multiple drugs are tried to treat PP, but as most have been studied in acute psychotic patients, it is often difficult to deduce whether the medication primarily treats the psychosis or the urge to drink.3, 5-7, 32 To prevent the development of hyponatremia, a number of supportive measures can be taken such as a balanced diet, avoidance of drugs that increase thirst, and frequent weighing to detect water retention timely. It is also important to recognize and treat predisposing factors for the development of hyponatremia such as drugs, comorbidities, and behavioral misconceptions.6, 7 The presence of hyponatremia should first be treated by fluid restriction. In the presence of extreme hyponatremia, 3% saline infusion can be considered, but beware that overcorrection may cause pontine myelinolysis.6
This case report shows that psychogenic polydipsia can present atypically with sudden onset of polydipsia and very large urine voids, mimicking CDI, and may occur in adolescents without prior behavioral problems. To differentiate PP from partial CDI, a water restriction test can be helpful when performed within the first week of onset. Although the water restriction test is the golden standard, newer alternatives such as arginine-stimulated copeptin measurements are easier and safer to use and have a higher diagnostic accuracy, in particular for differentiating partial CDI from PP.
ACKNOWLEDGMENTS
Published with written consent of the patient.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
Author 1 and author 5 drafted the initial manuscript. All other authors (KVM, JV, JDS) reviewed and revised the manuscript. All authors approved the final manuscript as submitted.
CONSENT FOR PUBLICATION
The patient and her parents provided written informed consent for publication, and the study was conducted in accordance with institutional guidelines.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.