A Japanese case of amoebic meningoencephalitis initially diagnosed by cerebrospinal fluid cytology
Abstract
Microscopy can detect the presence of amoebic trophozoites in cerebrospinal fluid and tissue. The infection was confirmed in the present case by polymerase chain reaction and immunohistochemistry, but we were unable to achieve a cure. Our case rapidly progressed without any skin lesions.
1 INTRODUCTION
Amoebic meningoencephalitis has an extremely high fatality rate. We recently suspected an elderly patient of having this rare infectious disease based on a cerebrospinal fluid test, cytology, and histopathology of a brain lesion following postoperative chemotherapy for lung adenocarcinoma. We ultimately detected Balamuthia mandrillaris by immunohistochemistry and polymerase chain reaction.
Amoebae can be classified into parasitic and free-living types. Parasitic amoebae feed on other animals, and Entamoeba histolytica in particular is known to cause amoebic dysentery in humans. Among free-living amoebae, more than 200 types are present in the soil and water in nature.1 While these protozoans have previously not been considered to be pathogenic in humans,1, 2 some have recently been shown to actually be parasites;1-3 for example, Acanthamoeba spp. causes amoebic keratitis, and B mandrillaris, Acanthamoeba spp., and Naegleria fowleri cause encephalitis.
Because the fatality rate of amoebic encephalitis is extremely high, the pathogen is known as the "brain-eating amoeba."4 In Japan, there have been only a few reports of amoebic encephalitis,5-9 and the route of infection, detailed clinical manifestation, and pathogenesis remain unclear.1
We recently experienced three patients whose cerebrospinal fluid (CSF) contained amoebae, and we herein report one case with amoebic meningoencephalitis. In this case, we detected amoebae in the CSF, and the pathogen was also found on cytologic specimens of the CSF. The pathogen was identified by immunohistochemistry and a polymerase chain reaction (PCR) analysis.
2 CASE PRESENTATION
The patient was a 63-year-old Japanese woman who complained of dyspnea and left recurrent nerve paralysis in March 2019. Her medical history included lung adenocarcinoma in the right upper lobe, which had been removed by thoracoscopic upper right lobectomy in 2016. The size of the lung cancer was 3.6 × 3.4 cm. Although regional lymph node metastasis was noted, no distant metastases or EGFR mutations were found (stage IIIA). She then received four courses of postoperative chemotherapy with cisplatin (CDDP, 55 mg/m2) and vinorelbine (VNB, 34 mg/m2). Twenty-six months after the chemotherapy, a ring-shaped nodule (Figure 1A) in the right occipital lobe was detected on head magnetic resonance imaging (MRI), suggesting brain metastasis of lung cancer. She received stereotactic radiosurgery, but the encephalopathic lesion continued to grow (Figure 1B). Therefore, six months after radiotherapy, enucleation of the encephalopathic lesion was performed to confirm metastasis. The resected brain tissue showed hemorrhagic, necrotic, and degenerated changes with lymphocytes-based inflammatory cell infiltration. However, we could not find any malignant cells in the tissue.
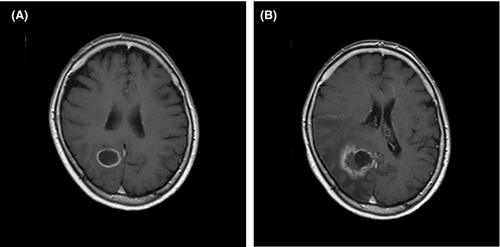
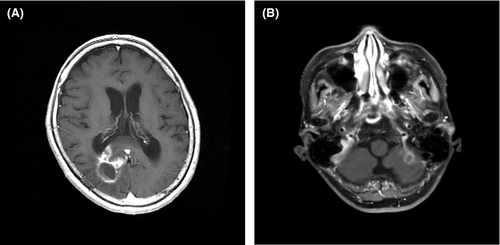
2.1 Head MRI three months after the removal of the encephalopathic lesion
One month after the removal of the encephalopathic lesion, her symptoms, such as hoarseness, recurrent nerve paralysis, and dyspnea, were not improved. Therefore, head MRI was performed three months after the enucleation of the brain lesion. The findings revealed endocranial and epidural invasion (Figure 2A) in the right occipital lobe and a new ring-shaped nodule (Figure 2B) in the left cerebellar hemisphere, suggesting meningeal carcinomatosis and cerebellum metastasis. A CSF examination was performed to confirm our clinical diagnosis.
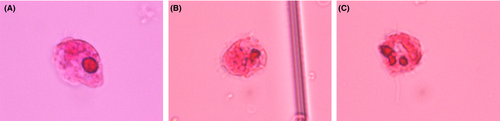
2.2 The CSF analysis
The CSF analysis showed severe increases in the protein level (135 mg/dL, reference range: 10-35 mg/dL) and numbers of mononuclear (20/μL) and polynuclear (16/μL) cells with an extremely low glucose level (2 mg/dL, reference range: 50-75 mg/dL). When the CSF was examined by a microscope after Sternheimer's staining, we observed many amoebae (Figure 3) with flagella that moved in a wave pattern and a few atypical cells.
2.3 Cytopathologic findings of the CSF
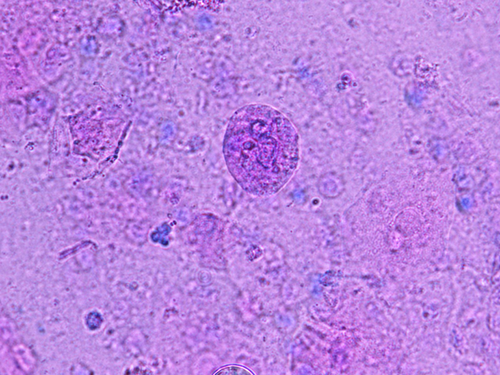
When cytologic specimens of the CSF were stained with the Papanicolaou and Giemsa methods, protozoa-like structures with weak light green-stained cytoplasm and multiple nuclei were found (Figure 4). In addition, a few atypical glandular cells (Figure 5) suggestive of adenocarcinoma were observed. These findings suggested amoebic meningoencephalitis and brain metastasis of adenocarcinoma.
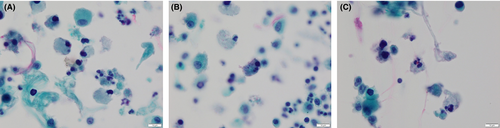
2.4 The examination of nasal discharge
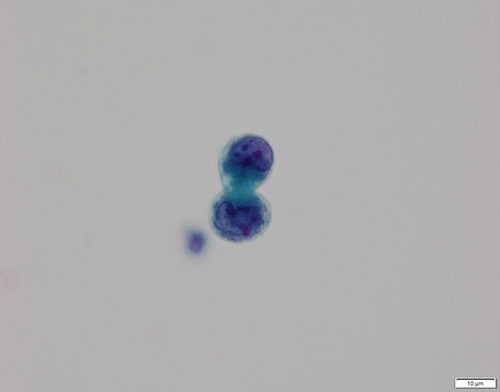
To determine the amoebic infection route, we obtained nasal discharge from the patient. Under the microscope, oval structures (possibly being dormant cysts) possessing a double outer-wall, and many protozoa with flagella movement (Figure 6) as found in the CSF were noted in the Sternheimer's stained nasal discharge.
2.5 Re-examination of the histopathology of the encephalopathic lesion
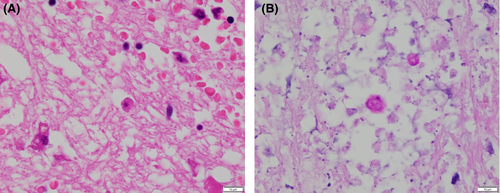
Formalin-fixed paraffin-embedded (FFPE) preserved specimens containing the encephalopathic lesion that had been previously extracted were re-examined by periodic acid-Schiff (PAS) staining to identify the amoebic infection in the lesion. Structures (Figure 7A) suggestive of small amoebic trophozoites (free-living form) were noted in the perivascular spaces and within the necrotic debris. The structures were positive for PAS reaction (Figure 7B).
The patient had a high fever (≥40°C) and dyspnea. We therefore diagnosed the lesion as amoebic meningoencephalitis and treated the patient with amphotericin B (100 mg/d), rifampicin (450 mg/d), fluconazole (200 mg/d), and albendazole (400 mg/d) for 2 weeks. However, the symptoms, including headache with a fever, dyspnea, and neck stiffness, were not improved, and she ultimately passed away approximately three months after amoebic detection. Consent to perform an autopsy was not obtained.
2.6 Immunohistochemistry and PCR analyses
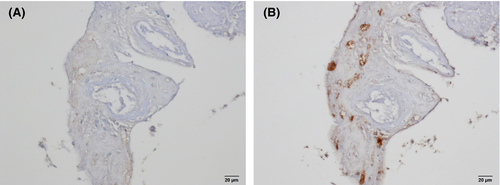
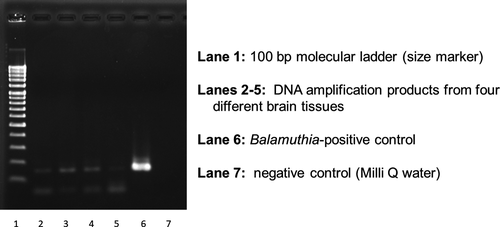
Immunohistochemistry and PCR analyses were performed by one of the authors (KY) to identify the pathogenic free-living amoebae in this patient. For this, FFPE tissues were prepared from the encephalopathic lesion that had previously been removed by operation. Immunohistochemical staining using anti-Acanthamoeba spp. and anti-B mandrillaris sera was performed on the FFPE tissue sections. While the tissues showed negative reaction to the anti-Acanthamoeba spp. serum (Figure 8A), they positively reacted to the anti-B mandrillaris serum (Figure 8B). Based on the findings of immunohistochemistry, pathogen-specific PCR using 18S rRNA gene DNA extracted from the specimen of brain tissues was performed after amplifying the 18S rRNA of the B mandrillaris genetic region. As shown in Figure 9, DNA amplification with the B mandrillaris-specific primer was detected on electrophoresis. We finally identified B mandrillaris as the cause of amoebic meningoencephalitis in this case.
3 DISCUSSION
In the past 50 years, more than 600 cases of amoebic encephalitis due to free-living amoebae, such as B mandrillaris, Acanthamoeba spp., and N fowleri have been reported around the world.1 Twenty-four cases of amoebic encephalitis have been reported in Japan,1 with the causative amoebae including 1 case of N fowleri, 4 cases of Acanthamoeba spp., and 18 cases of B mandrillaris.1 The cause in the remaining case is unknown.1
Encephalitis caused by free-living amoebae is roughly classified as primary amoebic meningoencephalitis (PAM) induced by N fowleri and granulomatous amoebic (meningo-) encephalitis (GAE) induced by B mandrillaris or Acanthamoeba spp.1, 10 While PAM frequently occurs in healthy young adults without underlying diseases, many cases of GAE are caused by opportunistic infections in immunocompromised patients.11 We herein report a case of amoebic meningoencephalitis that developed in a Japanese patient during radiotherapy and chemotherapy of lung cancer. We finally identified B mandrillaris infection by a PCR analysis and immunohistochemistry. We suspected that our patient was actively immunocompromised when she received chemotherapy and radiosurgery, since the white blood cell count, including CD4+, was less than 1000/mm3 (reference range, 3000 ~ 7800/mm3).
Balamuthia mandrillaris was first isolated from the brain of a mandrill baboon that died in the San Diego Zoo Wildlife Park of California in 1986, and the first case of human infection with B mandrillaris was reported in the same year.1, 3 Through skin wounds and nasal cavity, B mandrillaris present in the soil and water invades the body of patients with an immunocompromised condition due to an underlying disease or treatment.1 In general, ulcerated purple nodules in the skin are found in cases of infection with B mandrillaris through the skin.2 In our case, we detected B mandrillaris in the nasal discharge, but the patient did not have skin lesions. We therefore speculated that B mandrillaris had infiltrated her brain along the olfactory nerve from the nasal cavity and caused meningoencephalitis.
Most cases of amoebic encephalitis caused by B mandrillaris have been reported in the southern parts of North America and Latin America.3 B mandrillaris has reportedly been isolated in many samples from warm environments, such as a desert, tropical, or steppe climate. However, B mandrillaris was recently detected in the soil of Aomori, Japan, which is rather cold,12 suggesting that B mandrillaris can be found in the soil, regardless of the climatic zone. The life cycle of B mandrillaris has two stages: the vegetative trophozoite and dormant cyst stages.13 In the present case, we observed many protozoa with flagellar movement in both the nasal discharge and CSF. Dormant-like cysts were also found in the nasal discharge.
There have been few reports on amoebic meningoencephalitis in Japan, so the route of infection and detailed clinical manifestation are unclear.14 As such, no effective therapy against the disease has been established.14 In our hospital, we experienced three cases of amoebic meningoencephalitis (including the present case) over the six-month period from November 2018 to May 2019. All cases were caused by infection with B mandrillaris, suggesting that B mandrillaris is present in the living environment of Japan. Therefore, we should consider amoebic infection, including Balamuthia infection, when patients have infectious encephalitis, particularly those with elevated level of CSF protein, low level of CSF glucose, CSF pleocytosis (white blood cells > 5/mm3), and enhanced brain lesions on MRI.15 In addition, clinicians should be aware of Balamuthia as a cause of skin lesions and encephalitis.11, 16
The MRI, pathological, and cytological findings in this patient who developed GAE induced by B mandrillaris were similar to those reported previously.1 In our case, when considering brain metastasis of lung cancer, it was difficult to microscopically detect the presence of trophozoites in the perivascular necrotic area with infiltration of neutrophils in the brain lesion specimens, as previously mentioned.17 However, our re-examination revealed the presence of trophozoites, and B mandrillaris was detected immunohistochemically in the lesion.
PCR in conjunction with immunohistochemistry is a promising procedure for obtaining a fast and specific confirmatory diagnosis of GAE,18 resulting in the efficient treatment of this rare disease. To this end, a multiplex PCR assay has been validated as an important and specific tool for identifying, B mandrillaris, N fowleri, and Acanthamoeba spp. in clinical specimens,19, 20 although only a few reference laboratories are able to diagnose the disease.21
Light microscopy can be used to detect the presence of trophozoites in CSF and host tissue. Our diagnosis was ultimately confirmed by PCR and immunohistochemical analyses, but we were unable to successfully cure the patient because of the delay in the accurate diagnosis. In contrast to PAM, which is an acute, fulminant, rapidly fatal disease occurring in healthy children and young adults, GAE is considered to be a chronic or subchronic disease usually associated with previous skin granulomatous lesions. However, our case rapidly progressed without any skin lesions, as experienced in recent case reports.18, 22
4 CONCLUSION
Regarding the amoebic detection method, CSF testing with or without Sternheimer's stain that can confirm amoeboid movement is superior to other approaches. However, we can also observe the amoebic structures on cytological specimens, as in this case. The correct and early diagnosis of amoebic meningoencephalitis enable the administration of effective treatment. We therefore should carefully investigate the cytologic specimens through microscopy after obtaining a thorough understanding of this rare infectious disease as well as the physiology and structure of the amoebae. The recognition of this disease and specific diagnostic tests may lead to earlier and precise treatment, and ultimately reduce the severity and lethality of this infectious disease in humans.
ACKNOWLEDGMENTS
The authors would like to thank all the staffs in the Department of Parasitology, National Institute of Infectious Diseases for their help and support. Published with written consent of the patient.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
RA: collected the data, made the cytological diagnosis, and wrote the manuscript. TS: found amoebae in the CSF and took pictures and videos. AO, RN, MM, and FE: prepared the cytological and histological specimens and made the cytological diagnosis. NW: made the pathological diagnosis and provided PAS-stained and HE-stained pictures. KY: contributed to the immunohistochemical and PCR analyses and interpretation of data. TT: made substantial contributions to the interpretation of the data and manuscript review. All authors read and approved the final manuscript.