Trends, predictors, and outcomes of transcatheter aortic valve implantation in patients with bicuspid aortic valve related disease: Insights from the Nationwide Inpatient Sample and Nationwide Readmission Database
Assistant: Emma McCabe ([email protected]).
Abstract
Background
Transcatheter aortic valve implantation (TAVI) has increasingly been utilized in patients with bicuspid aortic valve (BAV) related aortic stenosis (AS) with insufficient large-scale data on its safety.
Methods
The Nationwide Inpatient Sample and Nationwide Readmission Database (2011–2018) were queried to identify patients undergoing TAVI for BAV versus trileaflet aortic valve (TAV) associated AS. The in-hospital, 30- and 180-day odds of outcomes were assessed using a propensity-matched analysis (PSM) to calculate adjusted odds ratios (aOR) with its 95% confidence interval (CI).
Results
A total of 216,723 TAVI (TAV: 214,050 and BAV: 2,673) crude and 5,347 matched population (TAV: 2,674 and BAV: 2,673) was included in the final analysis. At index admission, the adjusted odds of in-hospital mortality (aOR: 1.57, 95% CI: 0.67–3.66), stroke (aOR: 0.77, 95% CI: 0.38–1.57), cardiac tamponade (aOR: 0.75, 95% CI: 0.17–3.36), vascular complications (aOR: 0.33, 95% CI: 0.09–1.22), cardiogenic shock (aOR: 1.77, 95% CI: 0.93–3.38), paravalvular leak (aOR: 0.55, 95% CI: 0.26–1.14), need for mechanical circulatory support device, and permanent pacemaker implantation (PPM) (aOR: 1.02, 95% CI: 0.69–1.52) were not significantly different between TAVI for BAV versus TAV. At 30- and 180-day follow-up duration, the risk of stroke and major postprocedural complications remained similar, except that TAVI in BAV had a higher incidence of PPM implantation compared with TAV. The yearly trend showed an increase in the utilization of TAVI for both TAV and BAV and a steady decline in the overall annual rate of in-hospital complications.
Conclusion
TAVI utilization in patients with BAV has increased over the recent years. The relative odds of in-hospital mortality, and all other major complications, were similar between patients undergoing TAVI for BAV- and TAV-related AS.
1 INTRODUCTION
Bicuspid aortic valve (BAV) is increasingly recognized as a cause of accelerated aortic stenosis (AS) secondary to the advancement of imaging modalities. BAV is one of the most common adult cardiac congenital anomalies associated with severe AS, occurring in 0.5%–1.4% of the general population.1 Transcatheter aortic valve implantation (TAVI) has gained popularity as a feasible alternative to surgical aortic valve replacement (SAVR) in patients with trileaflet aortic valve (TAV) related symptomatic severe AS.2 However, BAV has remained a relative contraindication for TAVI as patients with BAV were excluded from all major TAVI trials in the United States (US).2-4 The exclusion of these patients was based on the unique features of BAV including asymmetric leaflet calcification and associated aortopathy.5 These anatomical characteristics spawned concerns for suboptimal results and possible TAVI-related complications including paravalvular leakage and aortic injury.6 Despite these reservations, reports from TAVI centers outside the US have shown acceptable feasibility and safety of contemporary transcatheter devices in patients with BAV.7 This has led to a recent paradigm shift with an increase in the off-label utilization of TAVI in patients with severe AS due to BAV. Although the number of BAV patients undergoing TAVI has been increasing, there is a paucity of large-scale data on the comparison of in-hospital and long-term outcomes of these patients. This study aims to provide evidence on the relative merits of TAVI in BAV compared to TAV from the largest available databases in the US.
2 METHODS
2.1 Data source
This retrospective study was conducted on the deidentified data obtained from the National Inpatient Sample (NIS) and National Readmission Database (NRD). Both NIS and NRD are publicly available databases, representing a similar cohort of the US population and are closely monitored by the Agency for Healthcare Research and Quality. These databases include information on 7 million hospitalizations/year, representing more than 100 million weighted discharges of national estimates. Both of these databases allow for nationwide estimates of hospital discharges of the same patient population, but they have important functional differences. The detailed index hospitalizations, trend analysis, demographic and baseline comorbidities data are available in the NIS. Using the special variable “NRD visit-link,” the NRD enables us to further identify the readmission encounters of the selected index cases. NRD lacks details on some of the demographic data such as race, geographical distribution, and regional variation of the disease that can be obtained from the NIS database. Institutional Review Board approval and informed consent are not required given the anonymized nature of data.
2.2 Selection criteria and outcomes
The nationally weighted 2011–2018 NIS and NRD claims were utilized to select all US adult patients (>18 years) who underwent TAVI for either severe BAV or TAV stenosis. The standard International Classification of Disease, Clinical Modifications codes (ICD-CM) were used to identify all patients. Baseline characteristics and in-hospital outcomes were collected using the ICD-9-CM and ICD-10-CM codes (Table S1). The primary outcome included in-hospital mortality and stroke. Secondary outcomes included procedure-related complications (cardiac tamponade, vascular complications, arterial rupture), major bleeding, paravalvular leak, cardiogenic shock, renal failure, and use of mechanical circulatory support (MCS) devices. The mean total hospitalization cost (adjusted for inflation index) and length of stay (LOS) were also compared between the two groups. The detailed definitions of all outcomes are given in Table S2.
2.3 Statistical analysis
The baseline characteristics between the two groups were compared using the Chi-square test for categorical variables, while the mean and standard deviation (SD) of continuous variables were analyzed using independent t test analysis or the Mann–Whitney U test. Cochran-Mantel Haenszel test was used to compute unadjusted odds ratios (OR) and its 95% confidence interval (CI) for outcomes at the index admission. Adjusted odds ratios (aOR) were calculated using a two-step approach; handling the missing values and obtaining a matched cohort with balanced demographics and baseline comorbidities. A Little's MCAR (missing completely at random) test was performed to differentiate systematically missing data (p < 0.05) from randomly missing data (p > 0.05). For randomly missing values trimming and winsorizing enabled to remove cases if the proportion of randomly missing values was <1%, while expectation-maximization accounted for >1% missing data. A 1:1 nearest neighbor stepwise multivariate propensity score matching (PSM) was performed for populations at each follow-up duration. The PSM maximum tolerated standardized mean difference (SMD) for significance was set at a caliper of 0.2 SMD. A total of 25 baseline elements were matched between the two comparison groups (Figure S1). Sensitivity analysis by the sequential exclusion of female, male, older adults, younger population, and those with no history of peripheral vascular disease (PVD) and coronary artery disease (CAD) was performed to assess its impact on pooled outcomes. For subgroup interaction analysis, a multivariate regression model was developed with an entry method using age-stratified variables and sex as potential moderators for major outcomes between TAVI for BAV versus TAV. A binary logistic regression model was performed to assess the predictors of in-hospital mortality in both groups. A type I error of p < 0.05 was chosen as a cutoff for statistical significance. All statistical analyses were performed using R 3.2, SPSS v24 (IBM Corp), and STATA v16 (StataCorp).
3 RESULTS
3.1 Selection of cases
Using NIS, a total of 216,723 TAVI-related weighted hospitalizations were included from the years 2011 to 2018, comprising 2673 BAV and 214,050 TAV patients with aortic disease undergoing TAVI. Using propensity-matched analysis, a matched cohort of 2,674 TAV patients was compared with 2,673 BAV patients.
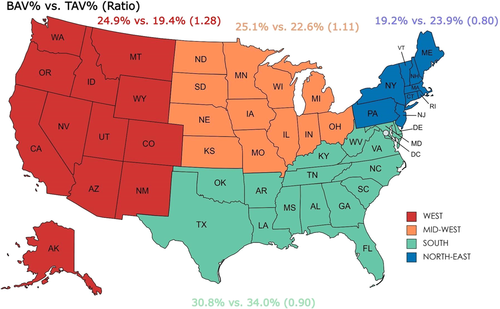
3.2 Baseline characteristics
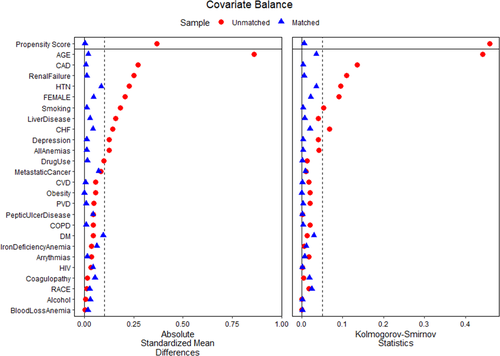
There were significant between-group differences in the demographics and baseline comorbidities of the overall population on crude analysis (Table 1). Patients undergoing TAVI for BAV were relatively younger compared with the TAV population (67.9 vs. 80.2 years; p = 0.02). Both groups were predominantly male and white. TAVI utilization for BAV was higher in the western regions of the US, compared with southern and northeastern regions (Figure 1). Comorbidities at baseline in BAV versus TAV groups included diabetes mellitus (11.2% vs. 12.6%, p = 0.005), hypertension (77.6% vs. 87.0%, p < 0.001), and atrial fibrillation (31.2% vs. 41.6%, p < 0.001) (Table 1). On propensity-score matched analysis, a matched population of TAV was selected with similar baseline comorbidities and demographics as BAV patients (Table 1). The mean Elixhauser comorbidity index for BAV (2.73 ± 1.58) was comparable to the TAV group (2.77 ± 1.66, p = 0.51). The propensity-matched variables and their SMDs are given in Figure 2. Major cardiovascular baseline characteristics on propensity-matched analysis are illustrated in Figure S2.
| Crude analysis | Propensity matched analysis | |||
|---|---|---|---|---|
| TAV (214,050) | BAV (2673) | TAV (2674) | BAV (2673) | |
| Age | 80.18 ± 8.4 | 67.91 ± 14.25 | 68.19 ± 14.4 | 67.91 ± 14.2 |
| Sex | ||||
| Male | 114,069 (53.30%) | 1668 (62.40%) | 1610 (60.20%) | 1668 (62.40%) |
| Female | 99,960 (46.70%) | 1005 (37.60%) | 1064 (39.80%) | 1005 (37.60%) |
| Race | ||||
| White | 177,314 (87.00%) | 2214 (86.70%) | 2209 (82.60%) | 2214 (82.80%) |
| Black | 8503 (4.20%) | 100 (3.90%) | 80 (3.00%) | 100 (3.70%) |
| Hispanic | 9597 (4.70%) | 80 (3.10%) | 170 (6.40%) | 80 (3.00%) |
| Asian or Pacific Islander | 2575 (1.30%) | 85 (3.30%) | 40 (1.50%) | 85 (3.20%) |
| Native American | 485 (0.20%) | <11 | <11 | <11 |
| Other | 5428 (2.70%) | 65 (2.50%) | 65 (2.40%) | 65 (2.40%) |
| Admission | ||||
| Weekday | 204,301 (95.40%) | 2554 (95.50%) | 2554 (95.50%) | 2554 (95.50%) |
| Weekend | 9749 (4.60%) | 120 (4.50%) | 120 (4.50%) | 120 (4.50%) |
| Region Old | ||||
| Northeast | 51,242 (23.90%) | 514 (19.20%) | 570 (21.30%) | 514 (19.20%) |
| Midwest | 48,441 (22.60%) | 670 (25.10%) | 600 (22.40%) | 670 (25.10%) |
| South | 72,737 (34.00%) | 825 (30.80%) | 959 (35.90%) | 825 (30.80%) |
| West | 41,629 (19.40%) | 665 (24.90%) | 545 (20.40%) | 665 (24.90%) |
| Category | ||||
| Government or private (collapsed category) | 1156 (0.50%) | 14 (0.50%) | <11 | 14 (0.50%) |
| Government, nonfederal (public) | 15,995 (7.50%) | 245 (9.20%) | 195 (7.30%) | 245 (9.20%) |
| Private, not-for-profit (voluntary) | 178,624 (83.40%) | 2250 (84.10%) | 2230 (83.40%) | 2250 (84.10%) |
| Private, investor-owned (proprietary) | 18,275 (8.50%) | 165 (6.20%) | 240 (9.00%) | 165 (6.20%) |
| Hospital | ||||
| Urban nonteaching | 20,334 (9.50%) | 240 (9.00%) | 220 (8.20%) | 240 (9.00%) |
| Urban teaching | 191,911 (89.70%) | 2434 (91.00%) | 2439 (91.20%) | 2434 (91.00%) |
| Small | 13,200 (6.20%) | 170 (6.40%) | 130 (4.90%) | 170 (6.40%) |
| Medium | 40,885 (19.10%) | 470 (17.60%) | 500 (18.70%) | 470 (17.60%) |
| Large | 159,964 (74.70%) | 2033 (76.10%) | 2044 (76.40%) | 2033 (76.10%) |
| Region New | ||||
| New England | 11,610 (5.40%) | 190 (7.10%) | 145 (5.40%) | 190 (7.20%) |
| Middle Atlantic | 39,140 (18.30%) | 315 (11.80%) | 425 (15.90%) | 315 (11.90%) |
| East North Central | 32,450 (15.20%) | 440 (16.50%) | 450 (16.90%) | 440 (16.60%) |
| West North Central | 15,760 (7.40%) | 230 (8.60%) | 150 (5.60%) | 230 (8.70%) |
| South Atlantic | 41,570 (19.40%) | 450 (16.80%) | 510 (19.10%) | 450 (16.90%) |
| East South Central | 11,635 (5.40%) | 155 (5.80%) | 150 (5.60%) | 155 (5.80%) |
| West South Central | 19,165 (9.00%) | 215 (8.00%) | 290 (10.90%) | 215 (8.10%) |
| Mountain | 12,750 (6.00%) | 280 (10.50%) | 185 (6.90%) | 280 (10.50%) |
| Pacific | 28,770 (13.40%) | 380 (14.20%) | 360 (13.50%) | 380 (14.30%) |
| Comorbidities | ||||
| Diabetes mellitus | 26,960 (12.60%) | 300 (11.20%) | 220 (8.20%) | 300 (11.20%) |
| Hypertension (HTN) | 186,288 (87.00%) | 2074 (77.60%) | 1979 (74.00%) | 2074 (77.60%) |
| HTN uncomplicated | 70,949 (33.10%) | 890 (33.30%) | 995 (37.20%) | 890 (33.30%) |
| HTN complicated | 116,674 (54.50%) | 1200 (44.90%) | 1005 (37.60%) | 1200 (44.90%) |
| Coronary artery disease | 147,288 (68.80%) | 1479 (55.30%) | 1470 (55.00%) | 1479 (55.30%) |
| Smoking | 8882 (4.10%) | 255 (9.50%) | 265 (9.90%) | 255 (9.50%) |
| Atrial fibrillation | 89,073 (41.60%) | 835 (31.20%) | 890 (33.30%) | 835 (31.20%) |
| Opioid use | 300 (0.10%) | <11 | <11 | <11 |
| Vasopressin use | 4563 (2.10%) | 70 (2.60%) | 45 (1.70%) | 70 (2.60%) |
| Myocardial infarction | 32,685 (15.30%) | 325 (12.10%) | 380 (14.20%) | 325 (12.10%) |
| Alcohol use | 727 (0.30%) | <11 | 15 (0.60%) | <11 |
| Cerebrovascular disease | 25,259 (11.80%) | 270 (10.10%) | 275 (10.30%) | 270 (10.10%) |
| Arrhythmias | 98,065 (45.80%) | 1180 (44.10%) | 1160 (43.40%) | 1180 (44.10%) |
| Heart failure | 157,691 (73.70%) | 1790 (67.00%) | 1735 (64.90%) | 1790 (67.00%) |
| Coagulopathy | 29,790 (13.90%) | 385 (14.40%) | 335 (12.50%) | 385 (14.40%) |
| COPD | 72,017 (33.60%) | 844 (31.60%) | 835 (31.20%) | 844 (31.60%) |
| Iron deficiency anemia | 8120 (3.80%) | 85 (3.20%) | 55 (2.10%) | 85 (3.20%) |
| Depression | 16,162 (7.60%) | 310 (11.60%) | 320 (12.00%) | 310 (11.60%) |
| Drug use | 925 (0.40%) | 45 (1.70%) | 40 (1.50%) | 45 (1.70%) |
| Electrolyte abnormalities | 38,604 (18.00%) | 569 (21.30%) | 550 (20.60%) | 569 (21.30%) |
| ESRD | 8178 (3.80%) | 45 (1.70%) | 135 (5.00%) | 45 (1.70%) |
| Hypothyroidism | 43,225 (20.20%) | 385 (14.40%) | 445 (16.60%) | 385 (14.40%) |
| Liver disease | 6851 (3.20%) | 195 (7.30%) | 215 (8.00%) | 195 (7.30%) |
| Lymphoma | 1619 (0.80%) | 20 (0.70%) | 50 (1.90%) | 20 (0.70%) |
| Metastatic cancer | 1270 (0.60%) | 45 (1.70%) | 70 (2.60%) | 45 (1.70%) |
| Neurologic disorder | 6518 (3.00%) | 85 (3.20%) | 85 (3.20%) | 85 (3.20%) |
| Obesity | 28,895 (13.50%) | 415 (15.50%) | 415 (15.50%) | 415 (15.50%) |
| Paralysis | 2235 (1.00%) | 40 (1.50%) | 35 (1.30%) | 40 (1.50%) |
| Peripheral vascular disease | 48,369 (22.60%) | 660 (24.70%) | 670 (25.10%) | 660 (24.70%) |
| Psychosis | 889 (0.40%) | 15 (0.60%) | 25 (0.90%) | 15 (0.60%) |
| Pulmonary circulation disorder | 41,024 (19.20%) | 540 (20.20%) | 440 (16.50%) | 540 (20.20%) |
| Renal failure | 77,733 (36.30%) | 680 (25.40%) | 665 (24.90%) | 680 (25.40%) |
| Rheumatoid | 9837 (4.60%) | 85 (3.20%) | 145 (5.40%) | 85 (3.20%) |
| Solid tumor | 4594 (2.10%) | 95 (3.60%) | 60 (2.20%) | 95 (3.60%) |
| Weight loss | 7762 (3.60%) | 140 (5.20%) | 125 (4.70%) | 140 (5.20%) |
| All anemias | 36,175 (16.90%) | 340 (12.70%) | 330 (12.30%) | 340 (12.70%) |
| Blood loss anemia | 2441 (1.10%) | 30 (1.10%) | 25 (0.90%) | 30 (1.10%) |
- Abbreviations: BAV, bicuspid aortic valve; COPD, chronic obstructive lung disease; ESRD, end stage renal disease; TAV, trileaflet aortic valve; TAVI, transcatheter aortic valve implantation.
3.3 Crude in-hospital outcomes of overall population
On unadjusted crude analysis, TAVI in BAV was associated with significantly higher odds of cardiopulmonary arrest, sepsis, ventricular tachycardia, cardiogenic shock, and the need for MCS, while TAVI for TAV had a higher rate of vascular complications and need for hemodialysis. The detailed outcomes with their respective odds ratios are presented in Table 2.
| Crude analysis | Propensity matched analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| BAV (2673) | TAV (214,050) | OR | p value | BAV (2673) | TAV (2674) | OR | p value | |
| Mortality | 70 (2.6%) | 4604 (2.20%) | 1.22 (0.96–1.55) | 0.11 | 70 (2.60%) | 45 (1.70%) | 1.57 (0.67–3.66) | 0.4 |
| Cardiac tamponade | 15 (0.60%) | 1739 (0.80%) | 0.69 (0.41–1.15) | 0.18 | 15 (0.60%) | 20 (0.70%) | 0.75 (0.17–3.36) | 1 |
| Vascular complications | 15 (0.60%) | 2110 (1.00%) | 0.57 (0.34–0.94) | 0.03 | 15 (0.60%) | 45 (1.70%) | 0.33 (0.09–1.22) | 0.15 |
| Cardiopulmonary arrest | 60 (2.20%) | 3361 (1.60%) | 1.44 (1.11–1.86) | 0.01 | 60 (2.20%) | 60 (2.20%) | 1.00 (0.45–2.25) | 1 |
| Sepsis | 65 (2.40%) | 2449 (1.10%) | 2.15 (1.68–2.76) | <0.0001 | 65 (2.40%) | 40 (1.50%) | 1.64 (0.67–3.99) | 0.38 |
| Stroke | 70 (2.60%) | 6894 (3.20%) | 0.81 (0.64–1.03) | 0.09 | 70 (2.60%) | 90 (3.40%) | 0.77 (0.38–1.57) | 0.59 |
| Major bleeding | 115 (4.3%) | 8928 (4.20%) | 1.03 (0.86–1.25) | 0.78 | 115 (4.3%) | 170 (6.4%) | 0.66 (0.38–1.14) | 0.17 |
| AKI | 350 (13%) | 27,446 (12.8%) | 1.02 (0.91–1.15) | 0.7 | 350 (13%) | 315 (11%) | 1.13 (0.78–1.62) | 0.58 |
| VT | 125 (4.7%) | 7397 (3.50%) | 1.37 (1.14–1.64) | 0.001 | 125 (4.7%) | 175 (6.5%) | 0.70 (0.41–1.19) | 0.23 |
| CS | 130 (4.8%) | 4905 (2.30%) | 2.18 (1.82–2.61) | <0.0001 | 130 (4.8%) | 75 (2.80%) | 1.77 (0.93–3.38) | 0.11 |
| PVL | 11 (0.4%) | 1969 (0.9%) | 0.40 (0.21–0.75) | <0.0001 | 11 (0.4%) | 20 (0.7%) | 0.55 (0.26–1.14) | 0.05 |
| Need for PPM | 270 (10%) | 20,093 (9.40%) | 1.09 (0.96–1.23) | 0.22 | 270 (10%) | 265 (9.9%) | 1.02 (0.69–1.52) | 1 |
| Need for IABP | 40 (1.50%) | 1798 (0.80%) | 1.79 (1.31–2.46) | <0.0001 | 40 (1.50%) | 25 (0.90%) | 1.61 (0.52–4.95) | 0.58 |
| Need for Impella | 25 (0.90%) | 600 (0.30%) | 3.36 (2.25–5.02) | <0.0001 | 25 (0.90%) | <11 | – | 0.07 |
| Need for ECMO | <11 | 255 (0.10%) | 1.57 (0.65–3.81) | 0.47 | <11 | <11 | 0.5 (0.05–5.52) | 1 |
| Need for HD | 30 (1.10%) | 4747 (2.20%) | 0.50 (0.35–0.72) | <0.0001 | 30 (1.10%) | 90 (3.40%) | 0.3 (0.2-0.5) | <0.0001 |
- Abbreviations: CS, cardiogenic shock; ECMO, extracorporeal membrane oxygenation; HD, hemodialysis; IABP, intra-aortic balloon pump; PVL, paravalvular leak; VT, ventricular tachycardia.
3.4 Propensity matched in-hospital outcomes
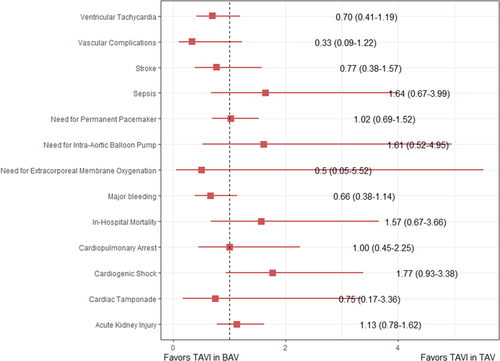
On propensity-matched analysis of 5,347 (2,673 BAV, 2,674 TAV) patients, there was no significant difference in the odds of in-hospital outcomes. The adjusted odds of in-hospital mortality (aOR: 1.57, 95% CI: 0.67–3.66), stroke (aOR: 0.77, 95% CI: 0.38–1.57), cardiac tamponade (aOR: 0.75, 95% CI: 0.17–3.36), major bleeding (aOR: 0.66, 95% CI: 0.38–1.14), paravalvular leak (aOR: 0.55, 95% CI: 0.26–1.14), vascular complications (aOR: 0.33, 95% CI: 0.09–1.22), cardiogenic shock (aOR: 1.77, 95% CI: 0.93–3.38), need for intra-aortic balloon pump (aOR: 1.61, 95% CI: 0.52–4.95), and PPM implantation (aOR: 1.02, 95% CI: 0.69–1.52) were nonsignificantly different between patients undergoing TAVI for aortic disease due to BAV versus TAV. The detailed list of outcomes is presented in Table 2 and Figure 3. There was also no significant difference in the mean adjusted cost ($56,840 ± 35,398 vs. $54,393 ± 28,791, p = 0.21) and length of hospital stay (5.96 ± 7.77 vs. 6.13 ± 7.33) between BAV and TAV, respectively (Figure S3).
3.5 Sensitivity analysis
A sensitivity analysis restricted to the younger population, older adults, males, females, and those with a prior history of CAD or PVD followed the findings of pooled analysis with a few important exceptions. Contrary to the net estimates, female patients and older adults had significantly higher mortality, while younger and male patients had a higher need for PPM with TAVI for BAV compared with TAVI for TAV. The visual illustration of bivariate analysis is presented in Figure S4. The risk of major bleeding was relatively higher in patients with TAV undergoing TAVI in a selected population of female patients, older adults, and those with PVD after exclusion of the male, younger and patients with no-PVD, respectively. The detailed proportion and effect sizes of major outcomes are presented in Table S3.
3.6 Subgroup interaction analysis
An interaction analysis to determine the impact of age and sex on major outcomes across TAVI for BAV versus TAV was performed. Older adults (p = <0.0001) and female patients (p = 0.0002) undergoing TAVI for BAV had a higher risk of in-hospital mortality compared with younger and male patients, respectively; and compared with similar patients (age >65 years and females) undergoing TAVI for TAV (Figure S5). Similarly, male patients had a significantly higher need for PPM implantation compared with female patients undergoing TAVI for BAV, as well as compared with male patients having TAVI for TAV. The detailed estimates of interaction analysis for all major outcomes are presented in Figures S6–S11 and Tables S4 and S5.
3.7 Trend analysis
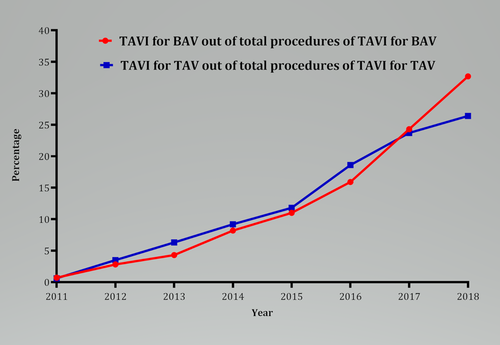
The yearly trends of the proportion of TAVI procedures performed for BAV and TAV are shown in Figure 4 and Table S6. Briefly, the trend shows an increased utilization of TAVI for BAV during recent years, contributing about 1.5% to the total number of TAVI procedures per year. More than 32% of TAVI procedures for BAV were performed during 2018. The yearly trend of outcomes showed a gradual decline in the overall rate of complications in both groups (Figure S12). The relative difference in the rate of complications between BAV and TAV was variable. Overall, the yearly trend of major bleeding, stroke, and mortality decreased, while the rate of PPM implantation remained high over the years in both BAV and TAV patients undergoing TAVI. However, the annual rate of PPM implantation with TAVI for BAV was significantly higher (p = 0.01), compared with TAVI for TAV (p = 0.07). The yearly trend of procedures and odds of outcomes are given in Tables S7–S8. The yearly trend of mean LOS and hospital cost between the two procedures are given in Figure S13 and Table S9.
3.8 Thirty- and 180-day adjusted outcomes
Using the NRD weighted estimates, a total of 19,031 and 23,975 post-TAVI rehospitalizations were identified at 30 and 180 days, respectively. The 30-day readmission rate of patients who underwent TAVI for TAV-related aortic disease was similar to those with BAV (OR: 0.88, 95% CI: 0.75–1.02), while the 180-day readmission rate for BAV was significantly lower (OR: 0.76, 95% CI: 0.65–0.88) than TAV. There was no significant difference in the 30- and 180-day odds of stroke, major bleeding, low degree heart blocks, cardiogenic shock and need for MCS devices between TAV and BAV groups. TAVI in BAV, however, was associated with a significantly higher incidence of third-degree heart block [(OR: 1.77, 95% CI: 1.18–2.65) and (OR: 1.55, 95% CI: 1.07–2.25)] and PPM implantation [(OR: 1.97, 95% CI: 1.36–2.93) and (OR: 1.9, 95% CI: 1.36–2.67)] at 30 and 180 days, respectively. The detailed estimates and proportion of outcomes are presented in Table 3.
| 30-Day outcomes | 180-Day outcomes | |||
|---|---|---|---|---|
| Outcomes | Odds ratio | p value | Odds ratio | p value |
| Readmission | 0.88 (0.75–1.02) | 0.11 | 0.76 (0.65–0.88) | <0.0001 |
| First HB | 0.84 (0.31–2.27) | 0.91 | 0.52 (0.19–1.39) | 0.25 |
| Second HB | 1.55 (1.03–2.32) | 0.04 | 1.43 (1.00–2.05) | 0.06 |
| LBBB | 0.80 (0.46–1.38) | 0.5 | 0.68 (0.43–1.07) | 0.11 |
| RBBB | 0.41 (0.10–1.67) | 0.29 | 0.88 (0.42–1.87) | 0.88 |
| CS | 0.95 (0.30–3.0) | 0.84 | 1.07 (0.44–2.58) | 0.93 |
| Third HB | 1.77 (1.18–2.65) | 0.01 | 1.55 (1.07–2.25) | 0.03 |
| Major bleeding | 0.80 (0.33–1.94) | 0.77 | 0.84 (0.43–1.62) | 0.71 |
| Stroke | 1.01 (0.41–2.46) | 0.83 | 1.2 (0.66–2.20) | 0.66 |
| Cardiopulmonary arrest | 0.84 (0.27–2.62) | 0.97 | 0.55 (0.18–1.73) | 0.42 |
| PPM | 1.97 (1.36–2.93) | 0.001 | 1.9 (1.36–2.67) | <0.0001 |
- Abbreviations: BAV, bicuspid aortic valve; PPM, permanent pacemaker; TAV, trileaflet aortic valve; TAVI, transcatheter aortic valve implantation.
3.9 Time to event and landmark analysis
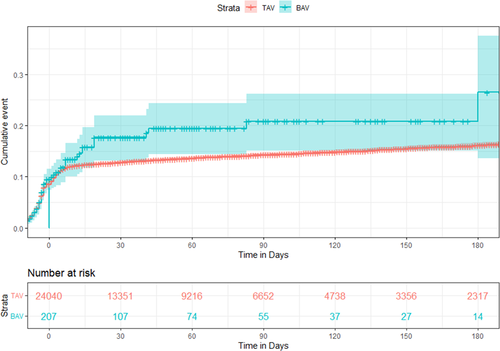
The Kaplan–Meier estimates of cumulative incidence of major outcomes are given in Figures S14–S16. Overall, there remained no difference in the incidence of AKI, stroke, and major bleeding points at all timepoints from index hospitalization till 180 days of TAVI. The need for PPM implantation was similar during the hospital stay but higher in BAV patients on all follow-up durations (Figure 5).
3.10 Predictors of major outcomes
The major predictors of mortality at the time of index admission are presented in Tables S10. Older age, female gender, cardiogenic shock, AKI, major bleeding, cardiac tamponade, and higher need of MCS devices were associated with a higher in-hospital mortality in patients with TAV, while cardiac tamponade, AKI, and Impella use were the positive predictors of mortality in patients with BAV.
4 DISCUSSION
The current study provides the most contemporary evidence on the safety of TAVI in appropriately selected patients with BAV using the largest available US databases. The major findings of our propensity-matched adjusted analysis include; (1). The cumulative complication rate in both groups was low (approximately 2%–9%). (2). There was no significant difference in the in-hospital adjusted odds of mortality, stroke, AKI, major bleeding, cardiogenic shock, need for MCS, and other procedure-related complications between both groups at index admission. (3). Older adults and female patients might have a higher risk of mortality with TAVI for BAV compared with younger and male patients, respectively, while male patients have a higher need for PPM implantation. (4). There was a rising annual trend in the utilization of TAVI in both groups, with a relatively greater increase in BAV patients. (5). The yearly trend of complications showed a decline in the risk of major bleeding events, while the annual rate of PPM remained high in both groups. (6). Postprocedure complications and increased use of MCS devices were associated with a higher risk of in-hospital mortality. (7). The incidence of procedural-related complications remained similar between the two groups up to 180 days of follow-up, except that patients with BAV have a higher risk of third-degree heart block and a higher need for PPM implantation on follow-up.
Historically, TAVI in patients with BAV has been avoided given the complex anatomy of the aortic valve, lack of large-scale evidence for safety, and limited operator expertize. The pivotal PARTNER and CoreValve trials excluded patients with BAV.3, 4 Therefore, BAV is a relative contraindication for TAVI in the recent guidelines.8 The major anatomical features that distinguish BAV from TAV include a higher incidence of heavy and asymmetric calcification of the fused raphe, large-sized and often noncircular annulus, the atypical position of coronary artery ostia, and a higher incidence of aortopathy with dilated aortic root.9 This, in conjunction with limited early operator experience and use of older generation devices, explains the worse in-hospital outcomes and higher incidence of procedural complications in the earlier studies of BAV.10, 11 The use of newer-generation TAVI devices with repositioning capacity and external sealing cuff for paravalvular leak prevention has likely resulted in more favorable outcomes and a rising trend in the utilization of TAVI in BAV patients.5 Furthermore, the widespread availability of TAVI, increasing operator experience, and expanding indications of TAVI enabled interventionists to intervene on patients with BAV associated severe AS who were previously deemed at high or unknown risk of complications.7, 12 This might explain our findings of higher utilization of TAVI in patients with BAV during 2017–2018 (57% of the total TAVI procedures for BAV) compared to early years.
The current study demonstrates a similar risk of in-hospital, 30- and 180-day stroke, major bleeding, and other post-TAVI complications between the two groups, except that PPM implantation was 1.5–2 times higher in patients with BAV. The latter finding could plausibly be explained by the complex anatomy, prolonged duration of the procedure, and increased paravalvular pressure due to balloon or mechanically dilated prosthetic valves in patients with BAV.13 To further determine the impact of baseline demographics on major outcomes, our interaction analysis showed that older adults and female patients appeared to have a higher risk of in-hospital mortality, while male patients had a higher need for PPM with TAVI for BAV. The former findings are possibly because of the higher burden of baseline comorbidities and frailty in older adults, while the higher incidence of PPM implantation in men could be explained by a greater requirement for larger size prosthesis (>25 mm) and increased procedure time leading to prolonged compression of the conduction system.13 In our study, the major cause for PPM implantation after the index TAVI procedure was delayed incidence of a third-degree heart block at follow-up.
The yearly trend of outcomes in our study showed a numerical decline in the annual rate of stroke, major bleeding, and mortality over the years. This is probably reflective of the enhanced operator skills, refinement of procedural techniques, and technological advancements of contemporary TAVI devices during recent years. The introduction of cardioembolic protective devices and the use of single antiplatelet agents instead of dual antiplatelet therapy post-TAVI might have contributed to the recent reduction in the stroke and major bleeding events, respectively.14, 15 A declining trend in TAVI-related mortality was also observed in both groups, possibly due to the expanding indications of TAVI to low-risk populations who have a higher life expectancy.3, 4 Based on these observations, the persistently higher annual rate of PPM requirement with TAVI might indicate inherent procedural-related injuries to the conduction system, rather than the operator's performance. Nonetheless, this did not translate into higher mortality as indicated by approximately 2% overall post-TAVI mortality in both groups.
Overall, our major results are similar to the findings of prior registries and a recent meta-analysis that showed a comparable risk of mortality in patients undergoing TAVI for BAV versus TAV.11, 16-18 Contrary to our results, an analysis of a subset of the transcatheter valve therapy registry reported a higher incidence of stroke and procedural complications in patients with BAV undergoing TAVI.6 The authors attributed this finding to the complexity of the procedure in the BAV group. However, a subsequent large-scale study demonstrated no difference in the incidence of major hard clinical outcomes between the two groups.5 Both these studies were limited to patients receiving newer generation balloon-expanding or self-expanding Evolut R or Evolut PRO valves and had no interaction, sensitivity, or trend analysis to determine the impact of potential moderators on outcomes. The more recent study by Makkar et al.18 also showed no difference in the short- and long-term difference in mortality and stroke between patients with BAV and TAV, however, these findings were limited to patients with low surgical risk. Given this, our large-scale adjusted analysis on all-comers irrespective of the type of device and surgical risk of the patients serves as a benchmark against which future results could be compared. Our interaction, sensitivity, time-to-event, landmark, regression, and trend analysis further identify the areas and timing of maximal benefits of TAVI in patients with BAV that were not reported by all of the prior studies. Table S11 outlines the major differences of our study from the previously published articles on the topic.
In summary, only 1.2% of all patients undergoing TAVI had BAV in our analysis. Despite the relative differences in the 30- and 180-day rates of PPM implantation and outcomes on subgroup interaction analysis, the overall rate of complications in both BAV and TAV was very low. This, in the context of the expansion of indications of TAVI to low-risk patients and the fact that 50% of patients undergoing SAVR have BAV, heart teams should seriously consider the feasibility of TAVI in patients with suitable anatomy.19 However, dedicated TAVI trials in comparison with SAVR in patients with BAV are needed to validate our findings.
5 LIMITATIONS
The results of our study should be interpreted in light of their limitations. The NIS and NRD are administrative claim-based databases that use ICD-10-CM codes for diagnosis that may vary in degree of detail and accuracy and are subject to misclassification. NIS has limited ability to distinguish comorbidities from in-hospital complications, therefore a well-validated and recommended Elixhauser comorbidity index was used to gauge the burden of comorbidities between the two groups.20 Within the HCUP databases, the Sievers classification of BAV types, generation of the prosthesis, valve type information, anatomic and echocardiographic characteristics of native valve anatomy is not recorded, and hence its potential effect on outcomes cannot be determined. This is a retrospective study, where patients undergoing TAVI were carefully selected based on their suitable anatomy, so residual confounding is possible, and selection bias could not be excluded. For the same reason, we could only report a temporal association between the TAVI procedure and in-hospital outcomes, and no definitive conclusions regarding the causation could be drawn. Although PSM is a well-accepted approach in an observational study to address differences in baseline characteristics and to obtain a balanced dataset, it cannot account for unmeasured or unknown confounding factors that might have impacted our pooled results. Similarly, we could not assess procedural characteristics such as balloon pre-dilation, prosthesis repositioning techniques, and duration and amount of contrast used in the procedure, which might have played a role in different outcomes. Although there was no direct assessment of the covariates such as operator skills and device type, the yearly trend in our analysis gave us an indirect measure of these estimates. Despite these limitations, this study remains one of the largest reported evidence on the safety of TAVI in BAV that can serve as a guide for future large-scale randomized trials.
6 CONCLUSION
The proportion of TAVI utilization in patients with BAV has increased over the recent years. The in-hospital, 30- and 180-day odds of stroke, major bleeding, and procedural complications are similar between patients undergoing TAVI for BAV- and TAV-related aortic valve disease. The relative risk of high degree block and need for PPM might be higher in patients with BAV at follow-up. Females and older adults have relatively higher mortality and male patients have a higher need for PPM with TAVI for BAV.
CONFLICTS OF INTERESTS
Dr. Deepak L. Bhatt discloses the following relationships—Advisory Board: Boehringer Ingelheim, Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Janssen, Level Ex, Medscape Cardiology, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, Stasys; Board of Directors: Boston VA Research Institute, Bristol Myeres Squibb, Society of Cardiovascular Patient Care, TobeSoft; Chair: Inaugural Chair, American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Boston Scientific (Chair, PEITHO trial), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Novartis, Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Chair, ACC Accreditation Oversight Committee), Arnold and Porter law firm (work related to Sanofi/Bristol-Myers Squibb clopidogrel litigation), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Cowen and Company, Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Piper Sandler, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today's Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CSL Behring, Eisai, Ethicon, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Javelin, Lexicon, Lilly, Medtronic, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Owkin, Pfizer, PhaseBio, PLx Pharma, Regeneron, Reid Hoffman Foundation, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, 89Bio; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald's Heart Disease); Site Co-Investigator: Abbott, Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Philips, Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, Takeda. Salim S. Virani: Research support: Department of Veterans Affairs, NIH, Tahir and Jooma Family. Honorarium: American College of Cardiology (Associate Editor for Innovations, acc.org). The other authors have no disclosures. Pinak F. Shah: Proctor: Edwards, Educational Grants: Edwards, Abbott, Medtronic, advisory Board: Xen
Open Research
DATA AVAILABILITY STATEMENT
The data supporting the finding of this study are available and within the article and its supplementary material. Raw data that support findings of this study are available from the corresponding author, upon reasonable request.