Fungal-Based Biorefinery: From Renewable Resources to Organic Acids
Abstract
Biorefineries are facilities in which lignocellulosic biomasses are converted in a wide range of bioproducts facilitating the transition from the use of petrochemical resources to renewable ones. Organic acids are considered very attractive for their utilization in different industrial areas as building blocks or as final bioproducts leading to a considerable market growth. They are metabolites which are naturally produced by microbials. The production of these molecules by filamentous fungi are attracting more attention due to their ability to hydrolyze lignocellulosic biomasses and to contextually produce different organic acids. Contrarily to a lot of other microorganisms, fungi have the ability to ferment pentoses, broadening the substrate utilization. The integrated use of lignocellulosic biomasses as material input and fungi as biocatalyst can contribute to make biorefineries more successful. This review gives an overview about the lignocellulosic biomass structure and hydrolysis, fungal morphology, and how they are connected. Further, it describes some relevant organic acids with regard to their processes, biocatalysts, industrial applications, and market considerations.
1 Introduction
In recent years, the overproduction of energy, chemicals, and synthetic materials from petroleum-based resources led to several environmental issues. In particular, pollutions, climate change, global warming, waste disposal, and natural resource reduction have reached devasting levels 1. Nowadays, all countries are gaining great attention to energy security, environmental concerns as well as to the development of alternative sources of chemicals 2. It is recognized that the use of fossil resources has several disadvantages, such as an unequal distribution on Earth, price vacillation, and not renewability. Taking together, all these characteristics make the use of fossil resources unsustainable for the future 3, 4.
In order to overcome the present and the future needs of humanity and to contrast the recent energy pandemic, biomass is emerging as an alternative renewable energy source for the production of fuels and chemicals 5. In this view, the use of the renewable raw materials and/or key components to produce different bioproduct(s) (bioenergy, biochemicals, biomaterials) was defined bio-based economy or bioeconomy. This new and greener economy model aims to replace fossil-based products by using the full potential of all types of sustainable sourced biomass due to its versatility, renewability and its carbon-neutral character 1, 6, 7. The exploitation of renewables results in the development of biorefineries. To be economically relevant, several and different bioproducts (high- and low-value products) must be produced at the same time.
Among the others, lignocellulosic biomasses (LCBs) are the most abundant natural carbon resource on Earth. They are typically supplied from agriculture (dedicated crops and crop residues), forestry (woody materials), industry (residues and wastes), domestic activities (organic residues), and aquaculture (algae, seaweed) 8. Because of the different sources, these feedstocks have different composition and proportion in terms of basic components: cellulose (30–50 wt %), hemicellulose (20–40 wt %), and lignin (15–25 wt %) 9, 10. Cellulose is a linear homopolymer consisting of glucose subunits, hemicelluloses are short-branched heteropolymers of pentose and hexose sugars, and lignin is a cross-linked polymer composed of three different substituted aromatic phenols 11. In order to convert biomass resources to biofuels and biochemicals, they are separated in their building blocks (i.e., carbohydrates) (Fig. 1) 8.
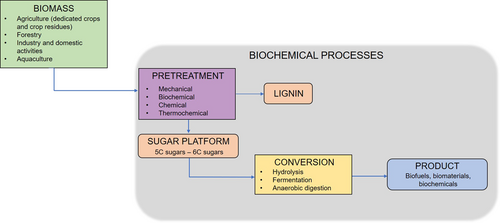
As mentioned above, biorefineries are able to transform feedstocks into biofuels as well as chemicals. Among the latter, organic acids are key products that can be produced by microbial processes either as natural products or as natural intermediates 12. Depending on the specific acid, many applications (e.g., food, pharmaceutical, cosmetic, and textile industry) can be proposed 13. Several attempts have been made to produce organic acids using raw material as substrate by either bacteria or fungi. Akerman et al. reported the production of acetic acid by Cellulomonas uda using brewers' spent grain as feedstock 14. Lactic acid was produced by Lactobacillus spp. on several raw materials, such as brewers' spent grain 15, 16, corn stover, and corncobs 17. Further, succinic acid was produced by Actinobacillus spp. using municipal solid waste 18, sugar beet pulp 19, and food waste 20, among others.
Traditionally, bacteria and yeasts have been employed as cell factories to produce bioproducts. However, filamentous fungi can be considered more suitable as biocatalysts in biorefineries. In particular, filamentous fungi are able to grow on low-cost substrate due to their ability to secrete a wide range of hydrolytic enzyme. Furthermore, they can ferment pentoses (mainly xyloses and arabinose) and are well recognized as major organic acid producers. Another aspect to take in consideration is the easy separation of the fungal biomass to the fermentation media, which make the downstream processing effortless. The recovered biomass of filamentous fungi can be further exploited due to the proteins and lipids contained, thus increasing the circularity of the process. All these statements enable to consider filamentous fungi ideal candidates in biorefineries 21-24.
Nonetheless, some limitations related to the use of filamentous fungi have to be mentioned. Firstly, the morphological complexity which causes problems during the fermentation step, especially for the mass and oxygen transfer limitation and the viscosity of the fermentation broth 25-28. According to this, scale-up processing results to be complicated 29. Furthermore, filamentous fungi have to observe some production process requirements. The biocatalyst has to be compelled to growth and to convert the biomass of interest. It has to produce the final bioproduct with high titer (50–100 g L−1), high yield (more than 0.5 g g−1) and high productivity (1.3 g L−1h−1) 13, 30. Lastly, the final bioproduct should be cheap to compete with the petroleum-based counterpart (about €2 per kg) 29, 31.
It is clear that wild-type microorganisms might be not able to ensure these assumptions. To overcome all these issues, genetic modifications in the biocatalyst as well as the development of a consolidated bioprocess (CBP) may represent possible solutions 13, 29, 32. CBP integrates enzyme production, biomass hydrolysis, and sugars fermentation in a single step 33, 34. According to this, CBP requires an efficient biocatalyst capable of producing efficient hydrolytic enzymes, consuming both pentoses and exoses and tolerating lignin-derived inhibitors 33, 35.
Several works about the biomasses, organic acid production, and recovery are available in literature 12, 13, 29, 36-40. However, the present review considers all the processes involved within a biorefinery platform. In particular, the aim of this review is to assess the production progress on the common organic acids by using filamentous fungi. Firstly, a short overview on the fungal morphology as well as on the influencing factors is discussed. Considering the general composition of LCB, the saccharification process and the advantages of using fungi on biomasses will be introduced. Afterwards, the biotechnological production routes of the most relevant organic acids will be presented, emphasizing the advantages and the features of these acids with respect to their industrial applications. Downstream strategies for organic acids recovery and refining are briefly discussed. Finally, techno-economic aspects, current bottlenecks in the commercial production of organic acids, and future perspectives are examined.
2 Fungi: Features and Morphologies
Fungi are eukaryotic organisms that naturally are able to (bio)degrade organic material. Filamentous fungi are structurally characterized by units called hyphae, which in turn can aggregate to form a mycelium 41. At this point, mycelia can be further differentiated into micro- and macroscopic morphology 42. The micromorphological fungal growth was described by Krull et al. who stated that the process starts from spores. Spores are quiescent structures which are not able to further move to other morphologies until the nutrients necessary for activation are supplied. Germination is the subsequent phase and it consists in the extension of the spores due to a swelling process. Finally, a singular germination tube emerges 43.
In submerged cultivations, the final macromorphology is affected by strain properties and cultivation conditions (e.g., inoculum properties, pH, cultivation temperature, and medium composition). All these parameters influence the final macroscopic morphology that can vary from freely dispersed mycelium to mycelial clumps and pellets (Fig. 2) 43, 44.
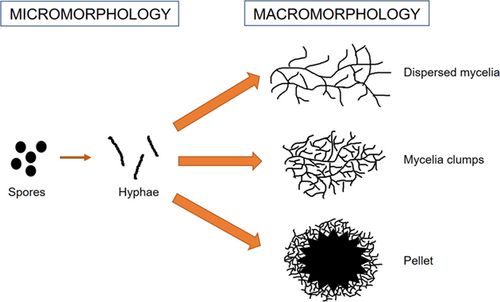
Pellets are spherical, branched agglomerates of several hyphae 43, 45. Two different types of formation processes are known: the coagulative and the non-coagulative. In the coagulative formation (e.g., Aspergillus spp.), the electrostatic and salt bridging between the growth media and the polysaccharides caused the thickening of the spores. At the end, the spores of the coagulative type turn in pellets. On the contrary, spores of the non-coagulative type (e.g., Rhizopus spp.) germinate before pellet formation. Some fungi, including Penicillinum chrysogenum, exhibit characteristics of both the coagulative and non-coagulative type: different hyphae are combined to form hyphal clumps which can further agglomerate to pellets 46, 47. Cairns et al. reported a hypothetic strategy for the formation of loose clumps. It could be possible that clumps are formed due to the culture conditions which disrupt or inhibit agglomeration 47.
It is recognized that the mycelial macromorphology is unpredictable since several factors affect this morphology 25. Among others, important factors include the carbon source and its concentration 48, nitrogen and phosphate limitation 49, pH 49, agitation 49, 50, spore inoculum level 51, oxygen enrichment 52, and osmolality 53.
Among the different filamentous fungi groups, Zygomycetes and Ascomycetes spp. have been employed in a wide range of industrial sectors 23. For instance, Ascomycetes have been employed in white biotechnology sectors for the production of antibiotics (Penicillinum chrysogenum) 54, enzymes (Aspergillus spp. and Trichoderma spp.) 55, pigments (Monascus spp.), and human food products (Fusarium spp. and Neurospora spp.) 56. Particularly, Aspergillus spp. are exploited for the commercial production of organic acids, which are considered relevant for the big market potential 23. Nowadays, due to the transition to the use of renewable biomasses, several feedstocks such as industrial wastes and lignocelluloses are employed for this purpose. In this regard, Ascomycetes are considered central biocatalysts for their ability to produce hydrolytic enzymes that can break down the recalcitrant structure of lignocellulosic biomass 23, 55.
3 Biomasses as Renewable Resources
Plants are mainly made up of lignocellulose that play an important rule from a structural point of view 57. Several parameters affect the chemical composition of LCB, such as plant species, maturity, climate, and environmental conditions 58, 59. In general, lignocellulose is composed of three main polymers: cellulose (40–50 %), hemicellulose (25–30 %), and lignin (15–25 %) (Fig. 3) 9, 10. They are usually associated with other minor components, such as pectin, proteins, extractables, and ash 60.
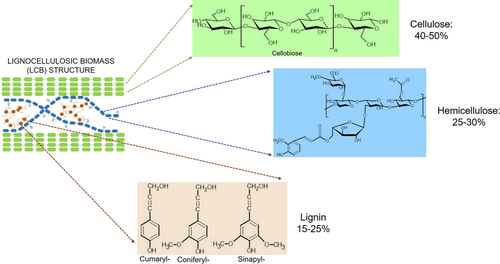
Cellulose is a linear polymer organized in D-glucose subunits, which are linked by β-1,4-glycosidic bonds 59, 61, 62. Cellulose is characterized by both crystalline and amorphous regions. Inter- and intramolecular hydrogen bonds and Van der Waals forces are found in the crystalline cellulose regions. On the contrary, amorphous cellulose is identified by a more casual structure due to weak bond linkages 63, 64. Hemicellulose is the second most abundant polysaccharide present in plants 63. It is characterized by less ordered and less resistant structure than cellulose 59, 62. Various polymers can be found in the hemicellulose, particularly pentoses (especially D-xylose and L-arabinose) and/or hexoses (D-mannose, D-glucose, and D-galactose) 63, 65.
Hydrogen and covalent bonds are the typical linkages in the hemicellulose. Further, hemicellulose is closely connected to both lignin by aromatic esters and to cellulose by hydrogen bonds, thus forming a bridge between cellulose and lignin 63. Lignin is an amorphous heteropolymer insoluble in water composed of three aromatic alcohols: p-coumaryl alcohol, coniferyl alcohol, and sinapyl alcohol. Each plant species has a different amount of these three units 63, 66-69. Furthermore, covalent, ester, and hydrogen bonds link the lignin to cellulose and/or hemicellulose 63, 70. All the structural components described above are responsible for the recalcitrant feature of LCB 63.
Filamentous ascomycetes such as Aspergillus spp., Fusarium spp., Monascus spp., and Neurospora spp. are interesting and versatile biocatalysts due to their ability to grow on a wide range of different substrates 23. In particular, filamentous fungi can consume carbohydrates released during the saccharification of lignocellulosic materials, such as hexoses and pentoses, and disaccharides such as cellobiose and sucrose 23, 71-73. Furthermore, the Ascomycetes can also grow on xylan, arabinan, and glucan 23, 56. Due to the secretion of different hydrolytic enzymes, filamentous fungi can be considered very flexible and adaptable. Indeed, the pattern of hydrolytic enzymes secreted depends on the substrate used 23. For instance, it was proved that filamentous Ascomycetes can use agricultural feedstocks such as wheat 74 and corn straw 75, wheat bran 72, rice hulls 311, sugarcane bagasse 76 or corn cobs 74. Further, filamentous Ascomycetes could also be used for valorization of agro-industrial waste streams including banana, orange, pineapple, carrots, anions and potato peels 23, 72, empty fruit bunches, or pomace 23, 77.
Several metabolites such as ethanol and organic acids have been produced by filamentous fungi using lignocellulosic feedstocks. Although the commercial production of organic acids is carried out by employing refined sugars, several attempts are currently in progress to use LCBs. The use of renewables from different sources represents an economically alternative that contributes to the development of circular economy 23, 55, 78. Consequently, as mentioned by Ferreira et al., the possibility to produce organic acids from LCBs would lower the overall production costs with simultaneous increase in the range of possible applications 23. Tab. 1 shows the feedstock used as raw material to produce the most prevalent organic acids by filamentous fungi.
|
Organic acid |
Substrate |
Microorganism |
Fermentation type |
Yield |
Operation scale |
Ref. |
|---|---|---|---|---|---|---|
|
Citric acid |
Apple pomace |
Aspergillus niger NRRL 567 |
SSF |
90 g kg−1 (DM) |
Shaking flask |
77 |
|
Banana peel |
Aspergillus niger UABN 210 |
SSF |
82 g kg−1 (DM) |
Shaking flak |
298 | |
|
Corncob |
Aspergillus niger NRRL 2001 |
SSF |
254 g kg−1 (DM) |
Shaking flask |
299 | |
|
Orange peel |
Aspergillus niger NRRL 599 |
SSF |
193 g kg−1 (DM) |
Shaking flask |
300 | |
|
Pomegranate peel waste |
Aspergillus niger B60 |
SSF |
351.5 g kg−1 (DM) |
Shaking flaks |
301 | |
|
Wheat straw |
Aspergillus niger ATCC 9142 |
SSF |
60.5 g kg−1 (DM) |
Shaking flask |
302 | |
|
|
Corn powder and citric acid effluent |
Aspergillus niger (industrial strain) |
SmF |
– |
Pilot scale |
103 |
|
|
Liquefied cassava chips |
Aspergillus niger 831 |
SmFa) |
0.82 g g−1 |
Shaking flask |
104 |
|
Fumaric acid |
Woody substrate hydrolysate |
Rhizopus delemar DSM 905 |
SmF |
0.39 g g−1 |
Shaking flask |
39 |
|
Aspergillus oryzae DSMZ 1863 |
0.58 g g−1 |
|||||
|
Dairy manure |
Rhizopus oryzae ATCC 20344 |
SmF |
0.31 g g−1 |
Shaking flask |
303 | |
|
|
Lignocellulosic syrup |
Rhizopus oryzae ATCC 20344 |
SmF |
0.43 g g−1 |
Shaking flask |
304 |
|
|
Corncob |
Rhizopus oryzae CICC 40351 |
Fed-batch SSFb) |
0.21 g g−1 |
Shaking flask |
305 |
|
Gluconic acid |
Waste paper |
Aspergillus niger IAM 2094 |
SmF |
0.92 g g−1 |
Shaking flask |
306 |
|
Corn Stover |
Aspergillus niger SIIM M276 |
SmF |
0.94 g g−1 |
Bioreactor |
188 | |
|
Itaconic acid |
Beech wood |
Aspergillus terreus DSM 826 |
SmF |
0.3 g g−1 |
Shaking flask |
307 |
|
Cellulose pulp |
Aspergillus terreus NRRL 1960 |
SmF |
0.62 g g−1 |
Shaking flask |
308 | |
|
Food waste |
Aspergillus terreus BD |
SmF |
0.27 g g−1 |
Shaking flask |
309 | |
|
Lactic acid |
Food waste |
Rhizopus arrhizus 2062 |
SmF |
0.97 g g−1 |
Shaking flask |
173 |
|
Malic acid |
Thin stillage |
Aspergillus niger ATCC 9142 |
SmF |
0.8 g g−1 |
Shaking flask |
310 |
|
|
Corn starch |
Aspergillus oryzae engineered |
SmF |
0.82 g g−1 |
Shaking flask |
202 |
|
|
Crude glycerol |
Aspergillus niger MTCC 281 mutant |
SmF |
– |
Shaking flask |
204 |
|
Succinic acidc) |
Birchwood chips and soybean hull |
Aspergillus niger Y-78 |
SSF |
32.5 g kg−1 (DM) |
Shaking flask |
221 |
|
Phanaerochaete chrysosporium A-381 |
||||||
|
Trichoderma reesei RUT-C30 |
- DM: dry matter; SmF: sub-merged fermentation; SSF: solid-state fermentation.
- a) Repeated-fed batch with cell immobilization.
- b) Fed-batch-SSF combined with separate hydrolysis and fermentation.
- c) Co-culture.
3.1 Saccharification of LCBs
Several hydrolytic enzymes are required to break down the LCB structure, such as cellulolytic enzymes (cellulases), hemicellulytic enzymes (hemicellulases), and delignification enzymes (lignases) 23, 79. The conversion of cellulose into glucose is led to the action of enzymes belonging to the glycoside hydrolase (GH) family with complementary activities 80. The complete hydrolysis of the cellulose requires three main groups of enzymes which work synergistically and concurrently 63, 81-83. Endoglucanases randomly cut β-1,4 glycosydic linkages in the less ordered regions of the cellulose thus releasing reducing and nonreducing chain ends; exoglucanases (also known as cellobiohydrolases) work at the end of the resulting chains producing cellobiose. The accumulation of cellobiose can lead to the inhibition of the cellobiohydrolases which, in turn, results in a lower cellulose conversion 84.
In order to complete the cellulose hydrolysis and to avoid cellobiohydrolases' inhibition, β-glucosidases are necessary. This last group of enzymes cleaves cellobiose into glucose units. More recently, it has been proven that the auxiliary activity of other enzymes is essential for improving the degradation of cellulose. Among these enzymes, lytic polysaccharide monooxygenases (LPMOs) play an important role in boosting the cellulose hydrolysis 85. LPMOs are believed to aid cellulose saccharification by creating nicks in the glucan chain on the amorphous regions of the cellulose microfibrils. These nicks provide new binding sites for the cellulolytic enzymes 24, 80, 86. The hydrolysis of the hemicellulose involves the action of several groups of enzymes due to the heterogenous nature and the presence of different side chains accordingly to the raw material 87.
Xylan is the most abundant constituent of hemicellulose characterized by β-1,4 linkages 88. The complete hydrolysis into xylose monomers is performed by xylanolytic enzymes which include both endo- and exo-xylanase (also known as β-xylosidase). The endo-xylanase acts on internal glycosidic bonds which results in the release of long and short xyloolisaccharides. The exo-xylanase cleaves the same linkages at the end of short xyloolisaccharides producing xylose monomers 9, 89, 90.
Mannan is made up of mannose residues or of a combination of mannose and glucose residues link together by β-1,4 bonds 87. β-Mannanase catalyzes the random hydrolysis of these linkages producing shorter chain of manno-oligomers with reducing and nonreducing ends. The activity of β-mannanase is sustained by β-mannosidase (exo-mannosidase) that work on the nonreducing ends to release mannose 80, 91. Due to the heterogeneity of the hemicellulose, other hemicellulases are required to completely hydrolyze the polymer. In particular, side chains can be cut from the backbone due to the activity of accessory enzymes such as α-arabinofuranosidase, α-glucouronidase, α-galoctosidase, and several esterase 91.
Lignin degradation is a more complex process which occurs via an oxidative process. Several enzymes are involved, namely laccases, manganese-dependent peroxidase, lignin peroxidases, and versatile peroxidases 80, 92-94.
4 Organic Acids Production and their Applications
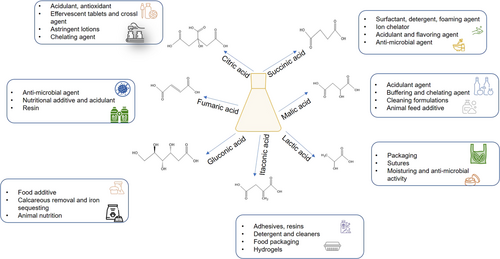
Organic acids are considered key building blocks chemicals with a large market. They are characterized by low molecular weight and one or more carboxyl groups (Fig. 4) 95. As reported by Mazzoli, the industrial application of organic acids produced by microbial processes strongly depends on their price, which represent the first limitation to their use 13. To expand the market of these organic compounds, the development of economically competitive bioprocesses is becoming imperative 13, 96. In this view, the production of bioproducts will ensure the development of a more sustainable economy, which is considered as a priority by the European Commission 13, 97.
4.1 Citric Acid
Citric acid (CA) has been the first organic acid industrially produced by wild-type and engineered recombinant fungi 95. CA is characterized by three carboxylic groups and is naturally found in citrus fruits. Although it can be produced by purely chemical reactions, microbial fermentations became the preference route of commercial CA production 98, 99. Currently, the global CA market is expected to reach USD 3.2 billion by 2023 and the global production is estimated to be around 736 000 tons per year 100. As stated by Show et al., due to low price and vast areas of application, it is evident that the demand of CA will be higher than the actual supply 99. For this reason, several efforts need to be carried out to optimize CA production, such as looking for alternative resources that are more economically, environmentally and productively convenient than current methods 99.
During the years, it has been demonstrated that the filamentous fungus Aspergillus niger is preferred with respect to other microorganisms for the commercial production of CA. The choice of Aspergillus niger is essentially due to higher production yield and its ability to consume cheap raw materials 99. The presence of some genes encoding for extracellular enzymes (invertase, glucoamylase, α-glucosidase, and α-amylase) make Aspergillus niger able to use various LCBs with different polymeric composition 29.
Nowadays, all industrial CA production is carried out via fermentation 101. Different fermentation processes have been developed: submerged fermentation (SmF), surface fermentation, and solid-state fermentation (SSF) 102. SmF is the most widely used fermentation strategy, especially in tank and bubble-column reactors 29. It ensures higher yields, higher productivity, less risk of contamination, and process standardization 29, 101, 102. Wang et al. reported the production of 157.3 g L−1 of CA in a batch reactor using corn powder and citric acid affluent as feedstock 103. Yu et al. produced 162.7 g L−1 of CA from liquefied cassava with a repeated-fed-batch strategy 104.
Surface fermentation was the first industrial technique used for CA production 104. The process is performed in trays assembled vertically (total capacity of 50–100 L) with temperature and humidity control. After the germination of the spores, the fungus starts to grow on the surface of the media as mycelial mat. Surface fermentation has several benefits due to the less energy costs, easy installation and operation processes 29, 101. Therefore, surface fermentation results to fit better in small and medium-scale industries 105. Generally, refined or crude sucrose, cane syrup, and beet molasses are the carbon sources employed 29, 101. SSF involves the utilization of a solid insoluble support that works either as source of nutrients or as physical support in a water-free environment 29, 101. It can be performed in different bioreactor configurations, i.e., flasks, trays, horizontal drums, and glass columns 101, 102, 106.
LCB materials can be valorized in SSFs, as they work as perfect substrates for fungal cultivations 105. Vandenberghe et al. produced 347 g kg−1 DM of CA using gelatinized cassava bagasse in flasks. Replacing flasks with column bioreactors, the authors obtained 309 g kg−1 DM of CA 107, 108. Soccol et al. described the production of CA in SSF using different raw materials, such as agro-industrial wastes, corncob, and wheat bran 105. Less consumption in terms of energy and water are among the main advantages of SSFs 102, 106. However, the process is not yet performed at industrial scale because of the difficulties in the standardization and longer time for CA recovery29, 101.
As reported by Di Lorenzo et al., several companies are carrying out CA biotechnological production using glucose and/or saccharose-derivate carbohydrates, such as Archer Daniels Midland-ADM (USA), Cargill (USA), COFCO Biochemicals (China), FoodChem (China), and Tate and Lyle (UK) 29.
Due to its biodegradability, its status of “GRAS” (“Generally Recognized As Safe” ), its versatility and its economic potential, CA has a wide range of applications 102. Mores et al. described the application of CA in the food and beverage industries, mainly as food acidulant to avoid the loss of organoleptic properties (with a purity of 99.5 %) 102. Cirimmina et al. stated the antioxidant ability since CA can chelate metal ions 109. In the pharmaceutical industry, CA is used as ingredient for effervescent tablets, as a palatability enhancer and as a part of film composition 102, 109-112.
Because of its low pH, CA is used also in the cosmetic industry in astringent lotions. CA finds applications in the chemical industry because of its ability to form complexes at neutral or low pH. Particularly, it is applied in electroplating, leather tanning, and metal oxides remover 102, 105, 111. Innovative areas of applications are emerging by year, such as in biodegradable packaging, antiseptic and extracting, and for (bio)remediation purposes 102, 109.
4.2 Fumaric Acid
Fumaric acid (FA) is a naturally occurring organic acid that is usually produced by involving chemical reactions. The chemical route requires butane, which is transformed to maleic anhydride. However, because of the increase of petroleum prices, the cost of the chemical precursor has increased as well. Further, the production of harmful by-products such as greenhouse gas strengthened the research of alternative processes 113, 114. Traditionally, the FA production has occurred via SmF and led to 4000 tons per year 29, 113, 115, 116. Later, the chemical synthesis prevailed and it is still the primary choice since it became economically advantageous and more efficient (111 wt % with respect to 85 wt % using microbial production from glucose) 114. However, due to the above-mentioned economic and environmental concerns, the fermentation-based production is gaining new attraction 29, 114.
The global market of FA has been reported to be 660.9 million USD and is projected to increase annually by 5.5 % (period 2021–2026) 117. Filamentous fungi from Rhizopus spp. are mainly involved in the production of FA, as they are able to produce FA under aerobic and anaerobic conditions 113, 118-122. SmF is the preferred fermentation strategy and is usually performed using glucose as carbon source 116. The production is carried out in batch reactors, although other configurations involving microorganism immobilization strategy are reported 116. Particularly, stirred tank reactors are the most used at industrial level, as they are easily to control and have low operation costs 116, 123.
The utilization of a batch reactor started with the Du Pont patent who reported a production of 121 g L−1 of FA 124. Roa Engel C.A. et al. produced FA in a tank reactor with oxygen and pH control reaching a yield of 0.28 g of FA per g of glucose 113. Kang et al. reported the production of 32.1 g L−1 of FA by Rhizopus oryzae in a batch reactor 125. Liu H., et al., used a batch slurry reactor to immobilize Rhizopus arrhizus on loofah fibers to produce FA from glucose 123. The productions of FA from renewables is still at lab-scale investigation. For instance, Das et al. studied the production of FA in SmF using two different substrates: apple pomace ultrafiltration sludge 126 and brewery wastewater 127. The authors obtained 25.2 and 31.3 g L−1, respectively 126, 127. LCBs are also used as substrate in SSFs. Liu X. et al. employed food waste as feedstock in a fed-batch SSF combined with a previous separate hydrolysis and fermentation strategy to produce FA 128.
In 2012, Xu Q. et al. reported that two Chinese companies, i.e., Changmao Biochemistry and Jiangsu Jiecheng Bioengineering, were focusing on the biotechnological production of FA at industrial level. However, Xu and co-authors stated that the fermentation process for FA production is still under development, as it results to be less efficient in terms of titer (30–50 g L−1) and productivity (0.4–1.0 g L−1h−1) with respect to the microbial production of CA, LA, and SA 115.
The industrial applications of FA are related to its structure: it has two carboxylic acid groups and a double bond between two carbon atoms 113. As reported by Yang et al. and Das et al., FA is mostly used in the food and feed industries as an antimicrobial agent, a nutritional additive, and acidulant agent due to its safety feature. Further, the hydrophobic nature of FA results in persistent, long-lasting sourness and flavor impact. Das et al. mentioned the application of FA in food products to buffer the pH, as it requires less amount compared to other organic acids due to its strength. Thus, the use of FA has the advantage to reduce the costs per unit weight 129, 130.
In the resin industry, FA is applied to assembly resins with different chemical nature 130. In particular, due to its chemical structure, FA is suitable for polymerization and esterification reactions 113, 130. As reported by Das et al., FA resins have the advantages to be more resistant to chemical corrosions (resulting in longer durability), and to be biodegradable and biocompatible, thus outcoming safer for environmental exposures 130. FA is also employed to produce biodegradable polymers, plasticizers, and carboxylating agents for rubber. Novel applications of FA esters are in the pharmaceutical industry for the treatment of psoriasis and sclerosis 29, 113, 131, 132.
4.3 Itaconic Acid
Itaconic acid (IA) is an interesting organic acid because of the presence of methylene groups conjugated double bonds, which give important chemical properties. Particularly, these groups allow polymerization through condensation and esterification with distinct co-monomers by carboxylic groups. According to this, IA is defined an innovative platform chemical suitable for monomer or during monomer synthesis 78, 133, 134. Nowadays, IA has a restricted market mainly because of to the limited assimilation of IA products and the presence of substitutes for those. The price of IA has changed during the years and is now stable in the range of 1.5–2.5 USD per kg 13, 135. However, as reported by Cunha Da Cruz et al., the possibility to decrease the production costs may contribute to the growth of the IA market 136. Nevertheless, the US Department of Energy has included IA into the leading twelve building block chemicals produced from renewable biomass 78, 137, with a market potential of 260 million USD in 2025 138.
Traditionally, the industrial production of IA occurred using different chemical routes: pyrolysis of CA, decarboxylation of aconitic acid, and oxidation of isoprene, among others 135, 137. Because of the long time, the low efficiency and the high costs, the chemical synthesis is losing interest 135, 139. Accordingly, the biotechnological route gained more attraction due to higher yield, production rate, and sustainability 135, 140, 141. Cunha Da Cruz et al. listed some of the main advantages of IA-related products with respect to other chemicals from fossil resources, such as its biodegradability (as homopolymer), non-toxicity, and the variety of possible derivates, among others 136.
IA is mainly produced by the fungus Aspergillus terreus due to its tolerance to low pH, its high yield, and titer of product 29. More recently, the natural producer basidiomycete Ustilago maydis gained attention as an alternative biocatalyst for large-scale production 29, 133, 142-144. Nowadays, the whole industrial production of IA is carried out via fermentation using glucose, simple sugars, or starch as carbon source reaching 41 400 tons per year 13, 29, 145, 146. De Carvalho and co-authors reported that the utilization of glucose as carbon source ensured a theoretical yield of 72 % with a final concentration of 86–129 g L−1 and with a productivity of 0.5–1.14 g L−1h−1 147.
SmF is the fermentation strategy widely applied either in laboratory or pilot scale using stirred-tank reactors and Aspergillus terreus as main producer 145, 148. Kuenz et al. used a 15-L tank reactor and obtained 86.2 g L−1 IA after seven days. The production yield reached 0.62 g of IA per g of glucose 149. Hevekerl et al. cultivated A. terreus in a 1.5-L stirred-tank bioreactor. They obtained 129 g L−1 of IA from glucose after almost five days of cultivation 150. Molnár et al. reported the production of IA using a 9-L stirred reactor with glucose as carbon source. At the end of the fermentation, the process yielded 0.85 g of IA per g of glucose 151. Kolláth et al. demonstrated the possibility to produce IA from xylose in a batch fermentation mode 152.
Because of the high shear stress caused by mechanical agitation which leads to cell damage, tank reactors might be not ideal for filamentous fungi 145. To overcome this issue, air lift reactors represent a suitable alternative for IA production, as it provides good mixing and oxygen transfer rates due to the liquid recirculation 145, 153. Using glucose as carbon source, Yahiro et al. produced 63.7 g L−1 of IA in an air lift reactor after seven days of cultivation 154. Other fermentation strategies are reported in literature, e.g., a fed-batch cultivation as reported by Krull et al. 155. Although glucose ensures the highest yield of IA, the development of process with the utilization of renewables is in progress.
At the moment, the use of lignocellulosic materials is still at lab-scale level 156-158. One of the major limitations is the presence of impurities that inhibit the IA production capacity of the fungi 29, 159. Recent studies are promoting continuous fermentations to improve the productivity 145. Several advantages led to continuous fermentation process: the possibility to maintain the optimal conditions during the whole fermentation period, the continuous product stream which simplifies the downstream process, and the circumvention of product's inhibition 145, 148. The industrial production of IA is concentrated in China, USA, and India 29, 139. In their recent review, Cunha da Cruz et al. listed the names of the companies currently involved in IA production 136.
Contrary to most of the other organic acids, IA is employed in non-food applications 95. It is relevant as building block for adhesives, unsatured polyester resins, finishing agents, detergent and cleaners, superabsorbent polymer, and dispersants 95, 136. Because of its trifunctional structure, IA allows the production of multiple novel biopolymers. These biopolymers are employed in different materials like intelligent food packaging, drug delivery, elastomers, hydrogels in water treatment, antibacterial biofilms, among others 78, 160.
4.4 Lactic Acid
Lactic acid (LA) is an organic acid widespread in nature. It exists in two enantiomeric forms because of the chiral carbon atom 38, 161. It is estimated that the LA market will reach 9.8 billion USD by 2025, with an annual growth rate of almost 19 % 162-164. LA can be produced by either chemical synthesis or microbial fermentation. The main disadvantage of the chemical route is related to the production of a racemic mixture of the L- and D-isomers. Moreover, this process involves the use of strong acids and lactonitrile hydrolyzed. In this view, biotechnological processes offer several advantages such as the production of optically pure L- or D-lactic acid (depending on the strain used), and the use of alternative raw materials as substrates 165.
Actually, more than 90 % of LA is produced via fermentation processes with a yield of 90–95 wt % according to the concentration of sugar used 29, 37, 166, 167. Refined sugars such as glucose and fructose have been traditionally employed for LA production 13, 166. These carbon sources were substituted with first-generation biomasses such as cassava, corn, molasses, and sugarcane 13, 166, 168. However, their utilization has been long debated as these feedstocks are in competition with the food/feed industry, thus causing ethical and economic issues 13, 37, 169. During the last years, several attempts have been made to shift the production of LA towards the utilization of nonfood sources, such as LCBs 166, 169. According to this, the use of low-cost raw materials would promote the development of competitive processes 161.
Although both fungi and bacteria are widely employed for LA production, the industrial production is actually ensured by lactic acid bacteria 29, 38, 170. Nonetheless, filamentous fungi have the main advantages to directly hydrolyze LCBs due to their ability to release saccharolytic enzymes, to tolerate low pH, and to simplify the separation of the fungal biomass from the fermentation broth 38, 169, 171. Fungi belonging to the genus Rhizopus attracted more attraction for their ability to produce the L(+) isomer of LA 161, 172-174. Among others, Rhizopus oryzoe is widely employed at lab-scale level by using immobilization techniques 168.
Efremenko et al. studied the production of LA using semi-batch conditions and with the immobilization on poly(vinyl alcohol)-cryogel 175. Tay and Yang immobilized R. oryzoe on cotton cloth using a repeated batch and fed-batch approach. The authors obtained 127 g L−1 of LA from starch and 226 g L−1 of LA from glucose 176. Sun et al. reported the immobilization of R. oryzoe on polyurethane foam cubes in an air lift bioreactor 177. Nowadays, it is estimated that the LA price was around 1.3-4.0 USD per kg and can vary according to the feedstock price and the final application; e.g., pharma and food required higher purity and this means more purification and refining steps 13, 37, 178. The main companies involved in LA supply are Cargill (USA), Galactic (Belgium), Corbion-Purac (The Netherlands), FKuR (Germany), and Shimadzu (Japan) 29, 37, 170, 179.
LA possesses a strong versatility as basic unit, which allows its use in many applications such as precursor of small (propylene glycol) or large (acrylic polymers) compounds 38, 165. The latter products exhibit important characteristics including biodegradability and biocompatibility. Accordingly, they are largely employed for labeling and packaging purposes. They can also be employed in the medical industry for manufacturing sutures and devices 38, 180. As reported by Du et al., most of the LA produced (about two-thirds) is used in the food industry to make yogurt and cheese 38. L-LA is the most common commercial form because the human body can better assimilate it.
With regard to LA, one of the most crucial processes is the polymerization to a highly crystalline polylactic acid (PLA). PLA was one of the earliest renewable chemicals presented on the market, particularly as biodegradable plastic, suitable for commercial use in fiber, film production, and food wrap 165, 181, 182. Also, PLA started to be present in hygiene and esthetic cosmetic products due to its moisturizing, antimicrobial, and rejuvenating action on skin 38.
4.5 Other Valuable Organic Acids
Gluconic acid (GA) is an organic acid produced from glucose through a dehydrogenation reaction conducted by the enzyme glucose oxidase 183. GA can be also produced by a chemical process; however, the fermentation process is the most used 183. Currently, the annual global market is between 50–80 million USD, and its industrial utilization is expected to pass 120 000 tons by 2024 184. The restricted market with respect to other organic acids is due to the high price of GA obtained through microbial production (about 1.20–8.50 USD per kg) 185.
The conversion of glucose to GA is usually conducted by the filamentous fungus Aspergillus niger. As reported by Ramachandran et al., A. niger is able to produce all the enzymes involved in the transformation of glucose into GA, including glucose oxidase, catalase, lactonase, and mutarotase 183. SmF is the most widely used process employed for GA production at industrial scale as it is highly efficient 184, 186. Glucose is one of the main carbon sources used and it is required at high concentrations 187. Since the fungal growth and the product formation are not related with each other 187, SmFs are carried out in batch reactors as reported by Zhang H. et al. 188 and Lu et al. 27.
Although the continuous fed-batch process is generally applied at industrial scale, it is not suitable for GA production by A. niger because of the high glucose concentration 184. Indeed, at high concentration of glucose, A. niger forms an excess of mycelia which in turn causes an increased viscosity of the media and a reduced aeration 183, 184, 189.
In recent years, new fermentation strategies with cells and enzymes immobilization are emerging. With regard to SSFs, only few studies have been reported. Roukas stated the production of 685 g kg−1 DM of GA from figs 190. Sharma et al. used sugarcane molasses as media and tea waste as solid support for the production of GA. After optimization, the authors obtained 82.2 g L−1 of GA 191. Singh et al. tested different fermentation strategies like SmF, surface fermentation, and SSF to produce GA from sugarcane bagasse. The authors found that SSF gave the higher GA concentration (106.5 g L−1) under the optimum time period 192. Among others, Jiian Biotech in India and Jungbunzlauer in Switzerland produce GA via fermentation.
GA is a noncorrosive, nonvolatile, nontoxic acid and it is a chelator agent at high pH values. Further, GA can be employed as plasticizer with good biodegradable properties (98 % at 48 h). Due to its properties, GA finds application especially in the food industry as a refreshing sour taster in wine and fruit juices. In the same sector, it can be employed also as component in meat and dairy products preparation. Moreover, as reported by Ramachandran et al., different salt forms of GA have various applications based on their features. For example, the sodium salt of GA acts as an anti-limescale on different surfaces. It is also used in the textile industry due to its ability to sequester iron at different pH values. On the other hand, the calcium salt form of GA finds application in the pharmaceutical industry as a nutritional supplement of calcium and in feed industry for animal nutrition 183.
Malic acid (MA) is a four-carbon dicarboxylic acid that can be produced by chemical, enzymatic, and biotechnological methods, where the first two routes are well established. In chemical synthesis, MA is produced from maleic anhydride, and the resulting product is a racemic mixture of D- and L-malic acid. Since maleic anhydride is produced exclusively from fossil resources, the need for a greener way is imperative. The current global MA production is around 80 000–100 000 tons per year, while the annual market demand is estimated to be more than 200 000 tons 12, 193, 194.
Two main advantages are reported for the microbial MA production: first, only the L-form of MA is produced and, secondly, microbial fermentations are performed by using a wide range of renewable substrates 195. MA is mainly produced by filamentous fungi belonging to Aspergillus spp. 196.The price of bio-based MA was evaluated to range from 1.80 to 4.4 USD per kg, but it is considered too high to be competitive with the petrochemical production 13, 28, 196. The production of MA via fermentation has not reached industrial production, thus only small-scale batch processes have been performed so far 195.
Glucose is the most used substrate which is generally needed at high concentration 197. For instance, Knuf et al. stated the production of 30.3 g L−1 of MA in a 2.7-L bioreactor using glucose 198. Recently, Shmitt et al. produced MA by a repeated batch cultivation with Aspergillus oryzae 199. By using this fermentation approach, Shmitt and co-authors obtained almost 178 g L−1 of MA 199. However, it was noted that the utilization of high concentration of glucose for MA production may cause the formation of the carcinogenic aflatoxins 197, 200. In addition, the utilization of glucose as carbon source makes the microbial production unsuitable to replace the chemical route production due to the high costs 195. Hence, the utilization of LCBs may represent a valid alternative 193, 195, 201.
Several studies reported the production of MA from renewables at lab scale. Liu et al. produced MA in SmF using shaking flasks co-fermenting glucose and corn starch. The authors obtained a yield of 0.82 g of MA per g of total carbon sources by employing an engineered strain of A. oryzae 202. Ochsenreither and co-authors obtained almost 40 g L−1 of MA from xylose in shaking flasks 203. Iyyappan et al. reported the production of MA from crude glycerol. In particular, the authors modified a strain of A. niger that was able to produce 77.4 g L−1 of MA after 192 h of fermentation in flasks 204.
The current application of MA is in the food and beverage industry where it is used as acidulant agent, often in combination with CA 195, 205, 206. MA is furthermore employed in the cosmetic industry, in personal care products, as well as in cleaning formulations due to its buffering and chelating properties 207. Other applications are in the pharmaceutical sector 195, in the semiconductor fabrication 207, 208, and as animal feed additive 209. Because of the presence of two carboxylic groups, MA represents an interesting building block compound for the synthesis of homo- and heteropolymers 210.
Succinic acid (SA) is characterized by two carboxylic acid groups. It has been traditionally produced from fossil-based resources, although many efforts are being made to use biotechnological processes. The total SA market is estimated to reach 94 000 tons by 2025, with an annual growth rate of 6.5 % 211. However, the SA production through fermentation processes is not competitive with the petrol-chemical route: the cost for SA produced using the petrochemical route is 2.5 USD per kg while for the biotechnological production is 2.94 USD per kg 29. Generally, SA is produced by bacteria isolated from rumen, such as Actinobacillus succinogenes 212, Anaerobiospirillum succiniproducens 213, Mannheimia succiniciproducens 214, and Basfia succiniproducens 215.
The production of SA from fungi is rarely described in literature. Nonetheless, some optimized fungal production systems are known to produce and to excrete the acid, such as Fusarium 216, Aspergillus 217, and Penicillium spp. 218. Moreover, literatures also reported the possibility to exploiting a co-culture of fungi and bacteria to obtain SA 219-221. In this latter case, SSFs were selected as fermentation strategy 20, 219. According to the biorefinery concept, few companies are able to produce SA at commercial scale, such as Myriant (USA), Reverdia (Italy), Succinity (Spain), and BioAmber (France) 29, 222-225.
As an important building block, there are four major applications of SA. It is used as a surfactant/detergent/extender and foaming agent. Further, SA is applied in food industry as acidulant/pH modifier, as a flavoring agent as well as antimicrobial agent 226. Finally, SA can be employed in the area of biodegradable plastics due to the possibility to be the precursor of a biodegradable polyester (polyethylene succinate) 12, 13, 227.
5 Downstream Strategies
The downstream of organic acids is one of the main limiting step in the biochemicals production 228. It is estimated that 50–70 % of the total cost comes from separation/purification process(es) 229, 230. Low acid concentration in the fermentation broth, difficult recovery technologies, utilization of harmful solvents, low purity and selectivity, and byproducts formation are the most diffuse downstream problems 228, 231. Furthermore, common difficulties are related to the complexity of the fermentation broth 232. A wide range of different methods have been developed to recover organic acids in fermentation broths such as precipitation 145, 233, membrane separation 230, 234, 235, and extraction 236, 237.
Other technologies such as distillation 228, 238, crystallization 239-241, and chromatography 241 are often used as a further purification step to refine the final acid 228. Nowadays, precipitation is the most employed recovery method at industrial scale, thus indicating its economic advantage with respect to the other strategies 228, 232. Therefore, further efforts need to be done in order to decrease the recovery costs and consequently the total costs of organic acids production in a biorefinery platform 237.
5.1 Precipitation
Precipitation has been the conventional method to recover organic acids from aqueous solution 237. It usually involves the use of calcium hydroxide at high temperature (up to 90 °C) to precipitate the calcium salt of the acid. The obtained insoluble salt form of the acid is separated from the liquid using a filtration step and then it is acidified with sulfuric acid 102, 228, 237. Finally, the acid can undergo to a further refining process, e.g., crystallization 228, 232. Precipitation is especially used for the recovery of CA 102, FA 242, 243, GA 244, IA 239, LA 245, and SA 224. Several advantages can be mentioned, such as higher selectivity, no phase transition, and high purity 228. However, major limitations are the formation of a considerable amount of solid waste and the production of calcium sulfate as side-product 102, 237, 239.
5.2 Membrane Separation
In recent years, membrane separation technology has attracted more attraction due to the high selectivity and adaptability 228. It involves the use of a membrane barrier to transport the compound of interest based on its permeability and gradient 228, 229, 239. Membrane separation can be pressure-driven or non-pressure-driven depending on whether a pressure is applied or not 234. In particular, based on the pressure levels, pressure-driven processes include microfiltration, ultrafiltration or nanofiltration 234, 246. These techniques have the great advantage of working continuously 229.
Membrane separation methods without any pressure involve inverse osmosis, pervaporation, and, more recently, electrodialysis 234, 247. This latter, in particular, permits the recovery with high yields and without solvent addition 230. Disadvantages of membrane separation processes are mainly due to the membrane fouling and clogging, high energy consumption, and high costs 229, 248. CA, FA, GA, IA, LA, MA, and SA are generally recovered via membrane separation techniques 242-244, 249-253.
5.3 Extraction
Extraction methods are based on the diverse solubility of the acid between two different phases 229. One of the most diffuse technique is the liquid-liquid extraction in which two immiscible liquids are used 229, 254. Typical organic solvent extractants are butyl alcohol, tributyl phosphate, and aliphatic amines 229. These extracts have incredible properties, such as the possibility to change the distribution coefficient, high efficiency, in situ process, low energy consumption, large scale of operation, and low cost due to the regeneration of the solvent 228-230, 254. However, they are not eco-friendly and have a limited use in the chemical industry due to the environmental standards 231.
In the last years, green alternatives have emerged for extraction techniques, i.e., bio-based solvents and ionic liquid solvents 231, 236, 255. Ionic liquid solvents involve the use of organic salts such as imidazolium, quaternary phosphate or quaternary ammonium salts 228, 256-258. Several benefits can be mentioned, such as chemical and thermal stability, low viscosity, non-volatile and non-flammable nature 228, 259, 260. Further, they are often considered as “designer solvents” since they can be adapted to different applications by mixing different ions. A wide range of organic acids can be recovered through extraction procedures such as CA, FA, LA, and SA 102, 116, 259, 261-264.
5.4 Distillation
Distillation is often used as refining step or in combination with other recovery methods, i.e., crystallization. Distillation acts by exploiting the differences in compound boiling points 265. By applying heat, a partial vaporization of the fermentation broth takes place 265. This in turn forms two phases (liquid and vapor) with different composition because of the diverse volatility of the components in the liquid phase 265. Although distillation is an efficient recovery technology since years, high costs are necessary to perform the process. Several studies have reported the application of distillation to separate LA from fermentative broth 238, 245, 262, 266, 267.
5.5 Crystallization
Crystallization is a typical purification step to produce a dry, stable, and solid organic acid 268. It can be performed under different conditions to obtain the final product in form of crystals with different sizes and morphologies 269. One main advantage is the possibility to recycle the reagent used for the crystallization, although it needs further chemicals and harsh conditions like low pH and high temperature 229. Moreover, this technology requires high energy input as well as a preliminary step to remove impurities 232. For these reasons, crystallization is a suitable method for larger amounts of fermentation broth. CA, IA, and SA are generally purified via crystallization 239, 270, 271.
5.6 Liquid Chromatography
Liquid chromatography is used as refining process 228. It requires the application of an adsorptive resin (stationary phase) which must be stable and insoluble in the solvents 228, 229. Ion-exchange resins are the most used to separate salts, sugars, and other organic molecules. The separation is based on the different affinity of the compounds to the resins. In particular, higher affinity means slower migration of the compounds along the resin bed 263, 272-274. Advantages are associated to the good selectivity, low energy requirements, no co-product formation, and higher yields. However, the management of the fermentative broth as well as the decrease of the exchange capacity are the main disadvantages 228, 275. This purification method is especially suitable for FA, IA, LA, MA, and SA 241, 243, 263, 272, 276.
6 Techno-Economic Aspects
In 2017, 224 active biorefineries were counted in Europe and the construction of new ones was already planned. About 181 of these operating biorefineries belong to the “first-generation” (1G) which employed sugar and starch-containing feedstocks, and only 43 biorefineries are “second-generation” (2G) biorefineries that work with lignocellulosic feedstock 277-279. Although LCBs are widely available, some challenges might be addressed to the supply and logistics 277, 280. Particularly, lignocellulosic feedstocks are subject to quality and quantity variability: since biorefineries operate all over the year, it is of great importance to ensure a continuous supply 277, 280, 281.
After harvesting, LCBs undergo compaction processes with the aim to homogenize the material in terms of sizes and weights 277. This step contributes to an efficient transportation and storage operation 277, 280. To overcome the challenges in the whole supply chain, the development of an integrated and centralized biorefinery facility represents a successful strategy 277, 282-284. This strategy is expected to reduce the costs related to the logistics and, consequently, the total production costs of a biorefinery 277, 284, 285.
Considering a biorefinery scheme, four other steps could be identified: a pretreatment to make the feedstock more accessible to the enzymes, the saccharification to hydrolyze the polysaccharides into sugar monomers, the fermentation to convert the monomers into final product(s), and the recovery 286-288. With regard to the pretreatment, several technologies have been developed 278. The choice of the pretreatment should take into consideration the efficacy, the scalability, the costs, and the environmental impact 277, 280. High energy requirement, large-scale feasibility, and formation of inhibitors affect the pretreatment costs as well as the whole conversion process 280. For instance, the formation of inhibitors directly influence the fermentation step, as these can inhibit the growth of the selected microorganism 278. To overcome this issue, a detoxification step might be required with an increase in the total costs 278, 289, 290.
Enzymatic hydrolysis has a great impact on a biorefinery chain. Generally, the utilization of enzymes at industrial scale is limited due to low availability since enzyme-producing microorganisms exhibit a low production yield of enzymes 280. Further, several saccharolytic enzymes are required to completely hydrolyze the biomass. Different solutions can be mentioned, such the “in-house” production 280, 284, 291, the recyclability through enzyme immobilization 280, 292-294, and the consolidated bioprocessing (CBP) 33, 280, 295.
As reported above, filamentous fungi are exceptional biocatalysts for the simultaneous production of enzymes and organic acids 21. In the next step of the conversion process of lignocellulosic feedstock to the final product, all sugars produced during the enzymatic hydrolysis must be consumed 211, 280. Actually, one of the major limitation is the consumption of both exoses and pentoses 278. Thus, recent studies are focused towards the research of wild-type or engineered microorganisms able to ferment all sugars produced and the possibility to employ a consortium of several microorganisms with different nutritional preferences 220, 278, 295. The recovery and the purification of the bioproduct(s) from the fermentation broth can be a very complex process that involves several technologies 280. Furthermore, to commercialize the final product, it must have a certain purity, especially for food and pharmaceutical applications 29. Hence, the cost related to the downstream processing is associated to the kind of the product recovered and to its final application 13.
Generally, the higher the number of the steps involved in a biorefinery scheme, the higher are the costs related to the facility 280, 296. One possible way to reduce the costs is the development of an integrated biorefinery in which multiple bioproducts are co-produced from different feedstocks 280. Accordingly, integrated biorefinery not only can generate both high-added value products and biofuels at the same time, but it can use the product of one feedstock as energy source for the other processes 280, 297.
7 Conclusion
Organic acids are important compounds with application as building blocks as well as final products in several industrial areas, such as food and feed, pharma, cosmetic, and polymers. Due to the wide range of uses, it is estimated that the worldwide organic acids market will reach about 35 billion EUR in 2027 29. Until now, the fossil-based production has significantly contributed to the rapid expansion of this market because of the low prices, the high conversion yields, and the high productivity. Nonetheless, critical concerns about the environmental, social, and economic impact might be assessed.
A concrete solution is represented by the bio-based economy in which renewable resources replace the fossil ones to produce the same goods or services. The overall idea of the bio-based economy is to promote the sustainability at all levels: a better quality of life in a conserved environment without any economic damage. The development of integrated biorefineries based on the use of different biomasses to produce a wide portfolio of bioproducts and the ability to be energy self-sufficient might be a key solution for the development of a circular bioeconomy in the next future.
In this review, the organic acids production by filamentous fungi has been analyzed for its potential to support the development of a bio-based economy. Some organic acids produced from first-generation biomasses are already on the market; however, speculations on their sustainability can arise. On the contrary, biochemicals from second-generation biomasses are not yet marketable. At the moment, the main limitations involve the considerable number of steps for the conversion of LCB into the final acid, the capability of the biocatalyst to metabolize complex substrates, the robustness of the biocatalyst, and the scale-up processing.
Genetic modifications to improve the biocatalysts' performances, either in the metabolization of the substrate or in the production of the final acid, are possible solutions. This is a common strategy adopted by the companies which generally work with genetically engineering strains. Moreover, until now, any discovered microorganism is able to ferment LCB to the final bioproduct in one step. Many efforts have been made to develop a single-step process, i.e., the so-called consolidated bioprocessing, in which the LCB is converted by the same organism. This novel process can potentially ensure an important reduction of about 40–77 % on the total costs of the process 13. Taking into consideration all the above-mentioned elements, more research toward the biotechnological production (from the upstream to the downstream processing) of organic acids must be carried out.
Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported by the German Federal Ministry of Food and Agriculture and the Agency for Renewable Resources (BMEL/FNR) through funding number 220NR026B. Open access funding enabled and organized by Projekt DEAL.
Abbreviations
-
- CA
-
citric acid
-
- CBP
-
consolidated bioprocessing
-
- DM
-
dry matter
-
- FA
-
fumaric acid
-
- GA
-
gluconic acid
-
- GRAS
-
generally recognized as safe
-
- IA
-
itaconic acid
-
- LA
-
lactic acid
-
- LCB
-
lignocellulosic biomass
-
- LPMO
-
lytic polysaccharide monooxygenase
-
- MA
-
malic acid
-
- PLA
-
polylactic acid
-
- SA
-
succinic acid
-
- SmF
-
submerged fermentation
-
- SSF
-
solid-state fermentation
Biographies

Ludovica Varriale is a Ph.D. candidate at the Technical University of Kaiserslautern. She is working in the group of Prof. Roland Ulber at the Department of Mechanical and Bioprocess Engineering. She received her M.Sc. in Industrial and Molecular Biotechnology in 2019 at the University of Napoli “Federico II”. She is currently working on the use of renewable resources to develop a green biorefinery platform. The research areas include pretreatments, enzymatic hydrolysis, and fermentations.

Roland Ulber is currently working as full professor at the Department of Mechanical and Bioprocess Engineering, Technical University of Kaiserslautern. He got his doctoral degree in 1996 at the University of Muenster and Hannover. In 2004 Prof. Ulber started his career as professor for bioprocess engineering at the Technical University of Kaiserslautern. Currently, his research activities are related to white biotechnology, biosensors for industrial applications, marine biotechnology, and electrobiochemistry.




