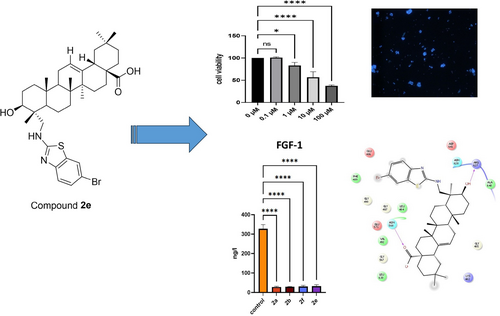
Design, Synthesis and Biological Evaluation of Novel Gypsogenin Derivatives as Potential Anticancer and Antimicrobial Agents
Abstract
Natural compounds are important sources for the treatment of chronic disorders such as cancer and microbial infectious disorders. In this research, Gypsogenin and its derivatives (2 a–2 f) have been tested against different cancer cell lines (MCF-7, HeLa, Jurkat and K562 cell lines) and further analyzed for cell proliferation, cell death type, and for act of the mechanism. Cell proliferation was determined by the MTT method and cell death types were analyzed with HO/PI staining. Fibroblast Growth Factor 1 (FGF-1), Interleukin 1 (IL-1), Interleukin 6 (IL-6), and Tumor Necrosis Factor Alpha (TNF-α), key players in breast cancer development and progression, were determined by Elisa kits. Results showed that compound 2 e inhibited the MCF-7 cell line proliferation with an IC50 value of 0.66±0.17 μM with 93.38 % apoptosis rate. Compound 2 e also decreased FGF-1, IL-1, IL-6, and TNF-α levels. Molecular docking studies performed in the binding site of FGFR-1 indicated that compound 2 e formed key hydrogen bonding with Arg627 and Asn568. Besides, compounds 2 a–2 f were evaluated for their antimicrobial activities against gram-negative and gram-positive bacteria and C. albicans via the microdilution method. Overall, compound 2 e stands out as a potential anticancer agent for future studies.
Graphical Abstract
1 Introduction
Semi-synthetic compounds derived from natural products always played a major role for cancer treatment. First defined compounds from plant have been used in oncology. Up to now, many products or its derivatives have been approved for chemotherapeutic agents.1-3
Especially, saponins are important bioactive compounds, mainly obtained from the roots of Gypsophila species.4-7 The triterpenoid saponins from Gypsophila plant species were reported to have remarkable biological activities, including anticarcinogenic,8, 9 anti-inflammatory,10 antitumor,11-13 antiproliferative,14 antioxidant activities.15, 16
Therefore, the roots that contain rich saponins of the Gypsophila species can be considered as a valuable source. These triterpenoid saponins can be obtained from Gypsophila plants representing the Caryophyllaceae family.17
Gypsogenin, a well-known natural saponin, exists abundantly in Gypsophila arrostii roots. Besides, gypsogenin (3-hydroxy-23-oxoolean-12-en-2-oic acid) has marked anticancer effects towards various types of cancer cell lines and our previous studies reported that gypsogenin aglycon and its derivatives have shown remarkable anticancer activities.18, 19 Due to pharmacological effects of gypsogenin and its derivatives, they have always attracted great attention. Especially, in recent years, studies on gypsogenin derivatives have increased due to the high potential of gypsogenin derivatives as important pharmacophores.20, 21
It is well-known that aniline derivatives exhibit a wide-range of biological activities. In addition, we reported that modifications at different positions of triterpenes resulted in higher anticancer activity.22 In another study, the C-23 aldehyde group was used to prepare different semi-synthetic compounds and these compounds were detected to possess a variety of pharmaceutical properties.23
Cancer is a complex and heterogeneous disease with multiple contributing factors involved in its progression. Fibroblast Growth Factor 1 (FGF-1), Interleukin 1 (IL-1), Interleukin 6 (IL-6), and Tumor Necrosis Factor Alpha (TNF-α) have emerged as key players in cancer development and progression. FGF-1, a member of the FGF family, has been shown to be dysregulated in breast cancer. It promotes angiogenesis, tumor growth, and metastasis by stimulating endothelial cell proliferation and migration. FGF-1 also influences tumor microenvironment by promoting the recruitment of stromal cells, including fibroblasts, which in turn enhance tumor cell survival and invasiveness.24 IL-1, an inflammatory cytokine, has been implicated in breast cancer initiation and progression. It promotes tumor cell proliferation, angiogenesis, and invasiveness through various signaling pathways. IL-1 also induces the secretion of other pro-inflammatory cytokines, creating a pro-tumorigenic microenvironment. Additionally, IL-1 contributes to the expansion of cancer stem cell populations, which are associated with treatment resistance and cancer recurrence.25 IL-6, another pro-inflammatory cytokine, plays a multifaceted role in breast cancer. It is involved in accelerating tumor growth, angiogenesis, and metastasis by stimulation of cell proliferation, survival, and invasion. IL-6 also contributes to immune evasion mechanisms employed by breast cancer cells. The elevated levels of IL-6 are associated with poor prognosis and resistance to therapy in breast cancer patients.26
TNF-α, a potent pro-inflammatory cytokine, is known to exert both pro- and anti-tumorigenic effects in breast cancer. Although TNF-α can induce apoptosis and inhibit tumor growth under certain conditions, persistent TNF-α signaling can augment tumor cell survival, invasion, and metastasis. TNF-α also influences the tumor microenvironment by modulating immune responses, angiogenesis, and extracellular matrix remodelling.27
Understanding the intricate interactions between FGF-1, IL-1, IL-6, TNF-α, and cancer is crucial for developing effective therapeutic strategies. Targeting these molecules or their downstream signaling pathways may provide promising avenues for the development of novel therapies aimed at combating breast cancer progression and improving patient outcomes. Further research is warranted to elucidate the precise mechanisms underlying their involvement in breast cancer and to explore their potential as therapeutic targets.
On the other hand, antimicrobial infectious disorders are one of the greatest challenges that we are facing today owing to the emergence of resistance to currently available antimicrobial agents. In particular, the resistance to antibacterial agents is apparently more crucial problem compare to the resistance to antifungal agents as human beings and fungal pathogens share similar clades in the tree of life and potential antifungals usually target the fungus and the host at the same time. Candida albicans, the most well-known opportunistic fungal pathogen, is responsible for lethal infections ranging from mucosal candidiasis to hematogenously disseminated disorders in particular vulnerable patients such as those with cancer or immune deficiency diseases. Due to the afore-mentioned issues, there is an extreme need to discover potent new antibacterial and antifungal agents to cope with the emergence of drug resistance.28-35
Many semi-synthesized compounds showed remarkable activity for their cytotoxic activities against various human cancer cell lines.36-40 In the current work, we reported a different method for the semi-synthesis of new gypsogenin compounds using new amine compounds. Then, we evaluated these derivatives for their anticancer and antimicrobial effects and further mechanistic mode of the anticancer activity.
2 Results and Discussion
2.1 Chemistry
The synthetic procedures performed in new semi-synthesis derivatives (2 a–f) was shown in Scheme 2. We carried out the semi-synthesis reactions of gypsogenin compound with different amine derivatives and we envisioned that target semi-synthesis compounds could be prepared by different method from our previous studies. Therefore, we tried new methods for new amine derivatives. Different amine derivatives were used for the targeted novel gypsogenin-amine derivatives (2 a–f). Firstly, starting compound (gypsogenin) was obtained from water extract of Gypsophila arrostii roots (Scheme 1).
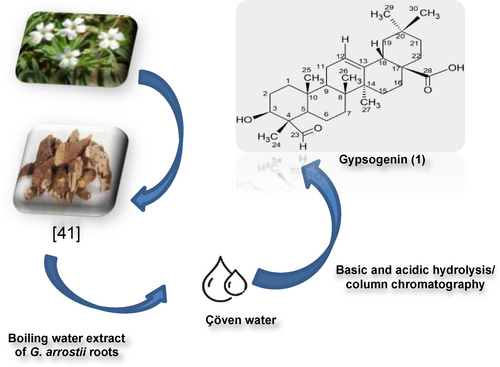
Obtaining of gypsogenin (1).
The semi-synthesis began with gypsogenin aglycon and different amine derivatives. All new semi-synthesis compounds occurred on the aldehyde group attached to carbon 23 (C-23) in the gypsogenin compound. Scheme 2 shows the intended new semi-synthesis compounds. The synthetic procedures were employed in dichloroethane under N2 atmosphere. The addition of sodium triacetoxyborohydride as a catalyst to the mixture under reflux reaction mixture led us to obtain final products (2 a–f). For all new semi-synthesis compounds, this reaction condition resulted in higher yields from 69 % to 85 %. The amine reduction reaction method using sodium triacetoxyborohydride and one drop acetic acid42 with reflux is different from from our previous work.43 It has become the focus of attention due to the fact that the final compounds were obtained with higher efficiency and the procedure was less costly. Moreover, the synthesized compounds showed higher yields.
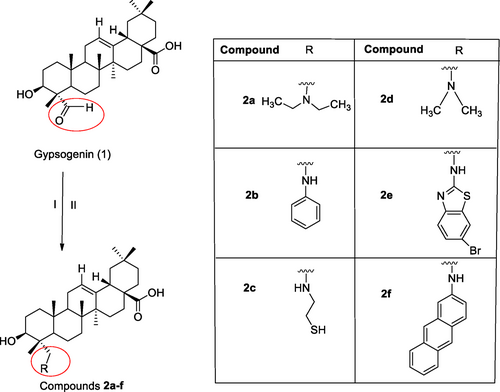
The synthetic route of final compounds 2 a–f. Reagents and conditions: (I) C2H4Cl2, amine derivatives, rt, N2 (g); (II) NaBH(OAc)3, CH3COOH, reflux, 2 h, N2 (g).
The purity of the semi-synthesis compounds was proven by IR, extensive NMR investigations (1H-NMR, 13C NMR) and LC–MS. Figure 1 shows the diagram for the spectral analysis.
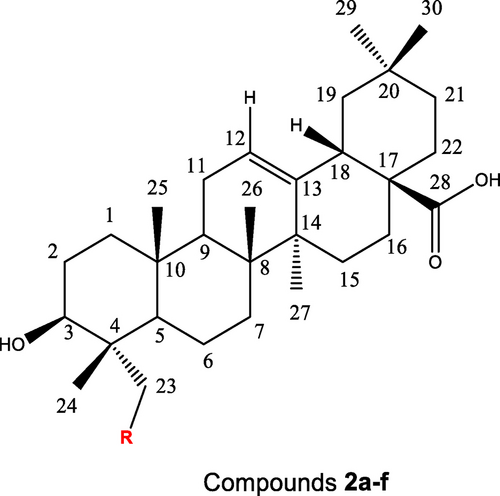
The diagram structure of new gypsogenin derivatives 2 a–f for spectral analysis.
The 1H-NMR spectra of all new compounds showed that six characteristic methyl peaks (H-24, H-25, H-26, H-27, H-29, and H-30) appeared at 0.50–1.04 ppm, 0.84–0.96 ppm, 0.68–1.03 ppm, 1.04–1.23 ppm, 0.85–1.77 ppm and 0.83–1.03 ppm, respectively. Therewithal, other specific peaks (H-3, H-12, H-18 peaks) were observed at 3.31–4.13 ppm, 5.13–5.48 ppm, and 2.70–3.30 ppm, respectively. The aldehyde proton signal (H-23; 10.75 ppm) of the starting compound (gypsogenin) disappeared in all final compounds. Instead, the signal for the aldehyde proton was converted to CH−N bond at 3.03–4.19 ppm (H-23). The 13C NMR spectrum of these novel semi-synthesis compounds indicated six methyl signals (C-24, C-25, C-26, C-27, C-29, and C-30) at 11.2–16.5 ppm, 13.1–17.8 ppm, 15.9–19.1 ppm, 25.7–26.6 ppm, 31.5–33.3 ppm, and 23.3–24.3 ppm, respectively. Other significant carbon signals (C-3, C-12, C-13, C-18, and C-28) appeared at 67.3–73.9 ppm, 121.9–123.1 ppm, 144.2–145.3 ppm, 41.0–46.9 ppm, and 179.0–184.3 ppm, respectively. The aldehyde carbon peak (C-23; 206.8 ppm) in the starting compound (gypsogenin) completely disappeared in final compounds. In the 13C-APT NMR spectrum, the C-23 for these new semi-synthesis compounds is easy to locate with a chemical shift as 46.1-64.6 ppm.
2.2 Biological Activity
2.2.1 In Vitro Cytotoxicity
Anticancer effects of prepared drugs on some cancer cells were outlined in Table 1. According to the MTT data, compound 2 e was identified as the most effective anticancer agent against MCF-7 cell line in this series with an IC50 value of 0.66±0.17 μM, indicating the importance of the presence of 6-bromo-1,3-benzothiazol-2-yl) amino moiety for the anti-breast cancer activity of gypsogenin derivatives. The efficacy of gypsogenin derivatives against breast cancer was followed by compounds 2 c (IC50=1.03±0.15 μM) and 2 a (IC50=2.40±0.82 μM). On the other hand, compounds 2 c and 2 a revealed the most significant anticancer effects against HeLa cell line with IC50 values of 0.74±0.12 μM and 1.88±0.73 μM, respectively. The anticancer effects of these agents were found to be higher than imatinib (IC50=3.03±0.26 μM). It was observed that compound 2 b was found as the most effective anticancer agent against Jurkat and K562 cells with IC50 values of 1.15±0.53 μM and 2.35±0.22 μM, respectively. The anti-leukemic effects of compound 2 b was found even higher than gypsogenin (1) and imatinib. This result pointed out that anilino moiety increased anti-leukemic effect of gypsogenin derivatives. Compound 2 e also displayed significant anticancer effect towards Jurkat cells with an IC50 value of 1.40±0.89 μM. In general, it can be concluded that almost all gypsogenin derivatives exhibited more significant anticancer activity than gypsogenin (1) and imatinib against all tested cell lines apart from K562 cells.
Compound |
IC50 |
|||
|---|---|---|---|---|
MCF-7 |
HeLa |
Jurkat |
K562 |
|
2 a |
2.40±0.82 |
1.88±0.73 |
4.12±0.72 |
13.23±3.12 |
2 b |
3.32±0.95 |
3.45±0.72 |
1.15±0.53 |
2.35±0.22 |
2 c |
1.03±0.15 |
0.74±0.12 |
6.15±1.51 |
8.42±3.51 |
2 d |
4.60±0.79 |
6.57±0.81 |
6.32±0.83 |
4.35±0.16 |
2 e |
0.66±0.17 |
5.24±0.74 |
1.40±0.89 |
3.56±1.42 |
2 f |
3.15±0.69 |
3.78±0.57 |
2.81±0.84 |
3.65±0.52 |
Gypsogenin (1) |
7.75±1.62 |
8.46±0.85 |
13.68±1.57 |
3.25±0.87 |
Imatinib |
3.19±0.71 |
3.03±0.26 |
13.92±1.87 |
4.60±0.66 |
- [a] IC50 values are presented as the mean±SD (standard error of the mean) from three separated experiment.
In addition, the compounds were examined against L929 cell line (healthy). Accordingly, it was found that these compounds did not have a dose-dependent toxic effect on L929 cells (Figure 2).
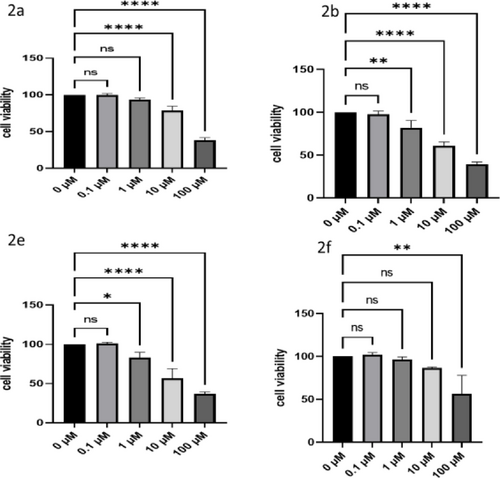
The dose-dependent toxic effects of agents on L929 (healthy) cells.
2.2.2 Apoptosis
After the MTT evaluation, apoptosis determination was made by considering the effective values. In this context, measurements were taken with HOPI monitoring after cell viability tests to determine whether the first applied agents played a role in apoptosis or necrosis cells in the effect groups. Accordingly, after drug cultivation in MCF-7 cells, the groups with the highest number of apoptotic cells were determined as compounds 2 e, 2 c, and 2 d with the values of 93.38 %, 88.34 % and 84.74 %, respectively. On the other hand, the groups with the highest number of apoptotic cells in HeLa cells were found as compounds 2 c, 2 a, and 2 f, respectively (92.45 %, 85.68 % and 83.68 %, respectively). This order was followed as compounds 2 e, 2 b, and 2 a for Jurkat cells (88.66 %, 87.07 % and 81.10 %, respectively), Finally, compounds 2 b, 2 f, and 2 e showed the most significant apoptotic activity against K562 cells with the values of 82.22 %, 80.79 % and 78.89 %, respectively. Briefly, the most significant apoptotic effects were detected against MCF-7 and HeLa cells by compounds 2 e and 2 c, respectively. These results were found totally compatible with MTT data (Table 2 and Figure 3).
Compound |
Cell Types (% apoptosis) |
|||
|---|---|---|---|---|
MCF-7 |
HeLa |
Jurkat |
K562 |
|
2 a |
82.55±1.02 |
85.68±1.24 |
81.10±2.93 |
72.12±0.64 |
2 b |
83.28±0.19 |
79.72±1.88 |
87.07±2.40 |
82.22±3.24 |
2 c |
88.34±5.08 |
92.45±1.67 |
73.29±4.11 |
74.05±4.95 |
2 d |
84.74±4.84 |
79.34±4.78 |
69.02±1.71 |
72.12±0.64 |
2 e |
93.38±0.66 |
77.01±2.40 |
88.66±1.30 |
78.89±3.89 |
2 f |
81.89±2.55 |
83.68±0.57 |
78.47±1.25 |
80.79±2.63 |
Gypsogenin (1) |
67.85±1.41 |
67.18±4.53 |
67.85±1.41 |
77.18±4.53 |
Imatinib |
58.28±4.3 |
58.23±3.22 |
61.62±0.94 |
61.57±3.36 |
Control |
2.78±1.42 |
3.09±1.67 |
2.78±0.37 |
3.42±0.58 |
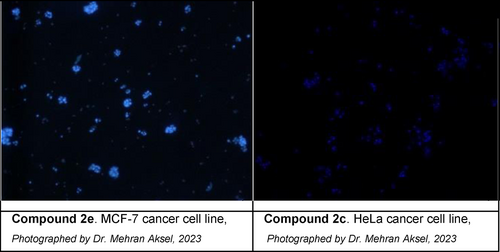
Apoptotic cells observed after application the compounds 2 e on MCF-7 and 2 c on HeLa cancer cell lines.
2.2.3 Pathway Assay
Our analysis revealed a significant decrease in the levels of FGF-1, IL-1, IL-6, and TNF-α in breast cancer patients compared to healthy controls (Figure 4). These findings indicated a potential dysregulation of the cytokine and growth factor network in breast cancer pathogenesis. The decreased levels of FGF-1 suggested a possible disruption of angiogenesis and cell proliferation pathways crucial for tumor growth and metastasis. Moreover, the reduced levels of IL-1, IL-6, and TNF-α pointed out altered inflammatory processes, which play a pivotal role in tumor progression and immune surveillance.
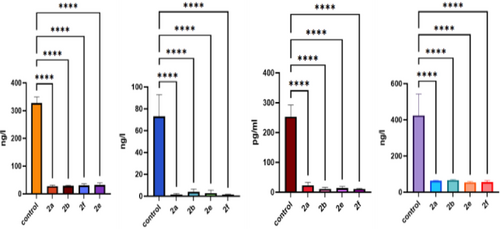
The levels of FGF-1, IL-1, IL-6 and TNF-α after agent treatment.
2.2.4 Antimicrobial Activity
The antimicrobial activities of gypsogenin and six new semi-synthesis gypsogenin derivatives were analyzed by the microdilution method and minimum inhibitory concentration (MIC) determination. Gram-negative bacteria (Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, Gram-positive bacteria (Stapylococcus aureus ATCC 25923, Bacillus subtilis ATCC 6633, and Entereococcus faecalis ATCC 29212), and yeast (Candida albicans ATCC 90028) were used for new gypsogenin derivatives.The results of antimicrobial activities of all compounds were listed in Table 3. According to the results, compounds 2 a–f generally showed low to moderate antimicrobial activity against bacteria strains and yeast. Compound 2 a inhibited the growth of Candida albicans ATCC 90028 with a MIC value of 125 μg/mL. Regarding the examining of the activities of new six semi-synthetic compounds against bacteria strains, it was revealed that compound 2 b demonstrated similar antibacterial effect with the main starting material gypsogenin aglycone against Enterococcus faecalis ATCC 29212 with a MIC value of 125 μg/mL.
Compound |
Test microorganisms |
|||||
|---|---|---|---|---|---|---|
Staphylococcus aureus ATCC 5923 |
Enterococcus faecalis ATCC 29212 |
Bacillus subtilis ATCC 6633 |
Escherichia coli ATCC 25922 |
Pseudomonas aeruginosa ATCC 27853 |
Candida albicans ATCC 90028 |
|
2 a |
>500 |
250 |
250 |
>500 |
>500 |
125 |
2 b |
>500 |
125 |
500 |
>500 |
>500 |
250 |
2 c |
>500 |
250 |
500 |
>500 |
>500 |
250 |
2 d |
>500 |
250 |
500 |
>500 |
>500 |
500 |
2 e |
250 |
250 |
500 |
500 |
>500 |
250 |
2 f |
250 |
250 |
500 |
250 |
>500 |
500 |
Gypsogenin (1) |
>500 |
125 |
>500 |
>500 |
>500 |
500 |
Streptomycin |
250 |
250 |
250 |
250 |
250 |
– |
Ketoconazole |
– |
– |
– |
– |
– |
250 |
2.2.5 In Silico Studies
Molecular docking studies were conducted for new gypsogenin derivatives (2 a–f) in the binding site of fibroblast growth factor receptor-1 (FGFR-1) compared to gypsogenin (1). Molecular docking studies were performed as supportive studies and this study is not based on computational studies. We performed molecular docking assessment to explore possible binding effects in the binding side of FGFR-1 and which interactions could be important and which substitutions could be more effective for future studies in optimization of gypsogenin derivatives (2 a–f). Results showed that compound 2 e revealed not only a different binding profile from gypsogenin (1) but also a similar binding profile with compounds 2 a and 2 b. Compound 2 f showed no significant interactions though it occupied the binding site region. Compound 2 e interacted through hydrogen bonding with Arg627 and Asn568 via its hydroxyl and carboxylic acid groups, respectively. Gypsogenin (1) presented a hydrogen bonding with Leu484 via its hydroxyl moiety. Compounds 2 a and 2 b also formed hydrogen bonding with Arg627 and Asn568. These results put emphasis on the importance of the presence of hydroxyl and carboxylic acid groups in new gypsogenin derivatives (Figure 5).
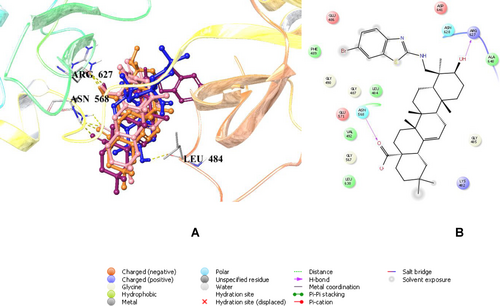
Docking poses of compounds 2 a, 2 b, 2 e and gypsogenin (coloured in orange, pink, maroon and blue, respectively) (A) and docking interactions of compound 2 e (B) in the binding region of FGFR-1 (PDB ID: 5UR1).
3 Conclusions
In summary, new gypsogenin derivatives (2 a–f) were synthesized and these new compounds were assayed for cytotoxicity activities against different human cancer cell lines. It was stated that compounds (2 a–f) with amine substituents at the C-23 position showed cytotoxic activity. Compounds 2 a–f exhibited significantly cytotoxic inhibition against MCF-7, HeLa, Jurkat, and K562 cell lines. It was observed from the results that compound 2 e was the most effective derivative against MCF-7 cell line (IC50=0.66±0.17 μM). Compound 2 c revealed the most effective anticancer activity against HeLa cell line with an IC50 value of 0.74±0.12 μM. Besides, the anti-leukemic effects of compound 2 b was determined to be highest against Jurkat and K562 cell lines and the IC50 values were calculated as 1.15±0.53 μM and 2.35±0.22 μM, respectively. The anticancer effects of compounds 2 a, 2 d, and 2 f were not found weak on all tested cancer cell lines but the most significant IC50 values of compounds 2 c and 2 e in particular against HeLa and MCF-7 cells, respectively encouraged us for further mechanistic experiments. The apoptosis ratios were found as 93.38 % in MCF-7 cell line for compound 2 e and 92.45 % in HeLa cell line for compound 2 c. Our study provides novel insights into the cytokine and growth factor milieu of cancer patients, revealing decreased levels of FGF-1, IL-1, IL-6, and TNF-α. Compound 2 e exhibited high binding affinity to the binding cleft of FGFR-1 based on the in silico studies with key hydrogen bonding. These findings suggested potential roles of these molecules in particular compound 2 e in cancer pathogenesis and warranted further investigation.
Experimental Section
The melting points of all compounds were measured with a Gallen camp electrothermal melting point instrument (uncorrected±0.1 °C). Infrared (IR) spectra were measured on a Perkin-Elmer Frontier FT/IR spectrometer. LC–MS was recorded on an Thermo Scientific/ Surveyor MSQ spectrometer. 1 H Nuclear magnetic resonance (NMR) spectra (600 MHz) and 13C NMR (150 MHz) spectra were recorded with an Agilent spectrometer. Column chromatography was carried out using 60 Å silica gel (Merck 7734). Thin-layer chromatography (TLC) analyses were carried out using 60 Å silica gel on F254 aluminium plates (Merck 5554). Fibroblast Growth Factor 1 (FGF-1; CAT NO: E0831Mo), Interleukin 1 (IL-1; CAT NO:E0191Mo), Interleukin 6 (IL-6; CAT NO:E0049Mo), and Tumor Necrosis Factor Alpha (TNF-α; CAT NO:E0117Mo) were purchased commercially from BTLAB (Shanghai Korain Biotech Co).
Plant Materials
Gypsogenin (1) was obtained from Gypsophila arrostii roots (Scheme 1) according to the previously described procedures.44 Structural characterization of gypsogenin and purity analyses were performed using TLC, NMR, and MS.
Chemistry
All solvents used were dried and distilled prior to use. Column chromatography (CC) was performed on silica gel using gradient eluent mixtures (dichloromethane:EtOAc (1 : 1) or hexane/ethyl acetate (8 : 2/1 : 1). Chemical shifts are reported in ppm relative to tetramethylsilane (TMS) (0 ppm) as reference. Data are reported as: chemical shifts, multiplicity (s=singlet, d=doublet, t=triplet, q=quartet, m=multiplet). Mass spectra were recorded using with an Thermo Scientific/ Surveyor MSQ spectrometer. The synthetic compounds were obtained with yields between 50 % and 87 %, and their structures were elucidated using full spectroscopic data.
Preparation of Gypsogenin (1) (3-Hydroxy-23-Oxoolean-12-En-28-Oic Acid)
After the commercially available water extract of Gypsophila arrostii roots, gypsogenin (3-Hydroxy-23-oxoolean-12-en-28-oic acid) (1) (Scheme 1) was prepared according to a literature procedure previously described.45 mp: 273–274 °C; LC/MS (ESI-MS) m/z=469.20 [M−1] (negative ion mode).
General Procedure for the Synthesis of the Gypsogenin Derivatives (2 a–f)
Semi-synthesis of novel gypsogenin derivatives (2 a–f) carrying different amine structures was performed as shown in (Scheme 2). Starting from the gypsogenin aglycon, gypsogenin derivative compounds (2 a–f) were synthesized. Initially, a solution of gypsogenin (1) (200 mg, 0.420 mmol) in dichloroethane (3 mL) was added amine derivatives (1.0 mmol) dissolved in dichloroethane under N2 atmosphere. The solution was stirred for half an hour at room temperature, then the sodium triacetoxyborohydride (approximately 2.0 mmol) and one drop acetic acid is added to the solution. After all this, the reaction mixture was kept on stirring under reflux at 2 h. TLC is used to monitor reaction completion. Approximately 1 hours later, the remaining borohydride is destroyed by the adding 20 mL of aqueous 5 % sodium bicarbonate solution. After the solution is placed in a separatory funnel, the aqueous phase is removed. The mixture was extracted with dichloromethane (10 mL×3). The collected organic layer was dried over anhydrous Na2SO4 (sodium sulphate) and concentrated in vacuo. The obtained organic phase was purified on silica gel column chromatography (hexane/ethyl acetate (6/4; 8/2) or dichloromethane/ethyl acetate (1/1)). The new gypsogenin derivatives (2 a–f) obtained in high yield were confirmed by various spectroscopic methods including IR, 1H-NMR, 13C-APT NMR, and LC/MS.
Synthesis of (3β)-3-Hydroxy-23-(Diethylamino) Olean-12-En-28-Oic Acid (2 a)
Column chromatography (dichloromethane/ethyl acetate (1/1)) gave pure 2 a as a yellow solid (87 %); Rf=0.6 (DCM/EtOAc 5 : 5); m.p. 237.3-238.6 °C; IR KBr (cm−1): 3443, 2924, 2848, 1642, 1456, 1380, 1262, 1027, 749; 1H-NMR (600 MHz, pyridine-d5): δ=0.92 (H-24), 0.96 (H-25), 0.99 (H-26), 1.03 (H-30), 1.04 (t, 6H, CH3CH2N−), 1.23 (H-27), 1.77 (H-29), 3.28 (dd, 1H, H-18), 3.71 (d, 1H, H-3), 4.19 (m, 1H, H-23), 5.48 (t, 1H, H-12); 13C-NMR (150 MHz, pyridine-d5): 14.5 (C-2a), 16.5 (C-24), 17.8 (C-25), 19.1 (C-26), 24.3 (C-30), 26.6 (C-27), 31.5 (C-29), 42.2 (C-18), 46.9 (C-1a), 61.8 (C-23), 68.4 (C-4), 68.5 (C-1a), 73.9 (C-3), 123.1 (C-12), 145.3 (C-13), 180.9 (C-28); LC/MS (ESI-MS) m/z Calc for C34H57O3 527.77 [M]− Found:527.80.
Synthesis of (3β)-3-Hydroxy-23-(Aniline) Olean-12-En-28-Oic Acid (2 b)
Column chromatography (hexane/ethyl acetate (8/2)) gave pure 2 b as a white solid (56 %); Rf=0.4 (DCM/EtOAc 5 : 5); m.p. 237.6–239.0 °C; IR KBr (cm−1): 3444, 2924, 2853, 1633, 1462, 1376, 1264, 742; 1H-NMR (600 MHz, pyridine-d5): δ=0.91 (H-29), 0.95 (H-25), 0.97 (H-30), 1.03 (H-26), 1.04 (H24), 1.20 (H-27), 3.29 (dd, 1H, H-18), 3.60 (1H, H-23), 3.72 (d, 1H, H-3), 5.48 (br s, 1H, H-12), 6.87 (NH), 7.20 (1H, H-2a/ H-6a), 7.57 (1H, H-3a/ H-5a), 7.90 (1H, H-4a); APT-NMR (150 MHz, pyridine-d5): 12.7 (C-24), 15.6 (C-25), 17.1 (C-26), 23.3 (C-30), 25.7 (C-27), 32.8 (C-29), 41.8 (C-18), 56.4 (C4), 67.4 (C-23), 73.1 (C-3), 122.6 (C-12), 123.6 (C-2a/C-6a), 131.0 (C-4a), 135.0 (C-3a/C-5a), 144.5 (C-13), 153.0 (C-1a), 181.2 (C-28); LC/MS (ESI-MS) m/z Calc for C36H53NO3 526.70 [M–COOH+Na+1]+; Found: 526.72.
Synthesis of (3β)-3-Hydroxy-23-[(2-Mercaptoethyl)amino] Olean-12-En-28-Oic Acid (2 c)
Column chromatography (hexane/ethyl acetate (6/4)) gave pure 2c as a white solid (79 %); Rf=0.4 (DCM/EtOAc 5 : 5); m.p. 218.5–219.5 °C; IR KBr (cm−1): 3434, 2648, 1650, 1457, 1265, 1209, 1031, 749; 1H-NMR (600 MHz, pyridine-d5): δ=0.90 (H-29), 0.95 (H-25), 0.98 (H-30), 1.03 (H-26), 1.04 (H-24), 1.22 (H-27), 1.96 (H-2a), 3.29 (dd, 1H, H-18), 3.73 (1H, H-23), 4.22 (d, 1H, H-3), 4.75 (H-1a), 5.48 (br s, 1H, H-12), 5.87 (NH−); APT-NMR (150 MHz, pyridine-d5): 12.9 (C-24), 15.7 (C-25), 17.1 (C-26), 23.3 (C-30), 23.4 (C-2a), 25.7 (C-27), 32.7 (C-29), 41.8 (C-18), 43.1 (C-1a), 67.4 (C-23), 67.5 (C-4), 73.0 (C-3), 122.5 (C-12), 144.5 (C-13), 180.0 (C-28); LC/MS (ESI-MS) m/z Calc for C32H55NOS 526.67 [M+Na+2]+; Found: 526.69.
Synthesis of (3β)-3-Hydroxy-23-(Dimethylamino) Olean-12-En-28-Oic Acid (2 d)
Column chromatography (dichloromethane/ethyl acetate (1/1)) gave pure 2 d as a white solid (63 %); Rf=0.4 (DCM/EtOAc 5 : 5); m.p. 251.0-252.6 °C; IR KBr (cm−1): 3434, 2924, 2853, 1644, 1415, 1382, 1275, 749; 1H-NMR (600 MHz, pyridine-d5): δ=0.90 (H-29), 0.95 (H-25), 0.98 (H-30), 1.01 (H-26), 1.04 (H-24), 1.22 (H-27), 3.30 (dd, 1H, H-18), 3.59 (N-(CH3)2(H-1a)), 3.72 (1H, H-23), 4.19 (m, 1H, H-3), 5.48 (br s, 1H, H-12); APT-NMR (150 MHz, pyridine-d5): 12.5 (C-24), 15.5 (C-25), 17.1 (C-26), 23.4 (C-30), 25.7 (C-27), 32.9 (C-29), 43.1 (C-18), 48.7 (N-(CH3)2(C-1a)), 67.7 (C-23), 70.4 (C-4), 73.3 (C-3), 122.3 (C-12), 144.6 (C-13), 184.3 (C-28); LC/MS (ESI-MS) m/z Calc for C32H55NO 453.20 [M–CH3+1]+ Found: 453.22.
Synthesis of (3β)-3-Hydroxy-23-[(6-Bromo-1,3-Benzothiazol-2-Yl)amino] Olean-12-En-28-Oic Acid (2 e)
Column chromatography (dichloromethane/ethyl acetate (1/1)) gave pure 2 e as a yellow solid (87 %); Rf=0.39 (DCM/EtOAc 1 : 1); m.p. 116.2–117.2 °C; IR KBr (cm−1): 3440, 2920, 2844, 1640, 1455, 1290, 1255, 1027, 749; 1H-NMR (600 MHz, pyridine-d5): δ=0.50 (s, H-24), 0.68 (s, H-26), 0.85 (s, H-30), 0.85 (s, H-25), 0.86 (s, H-29), 1.04 (s, H-27), 2.72 (dd, J=6.8, 13.4 Hz, H-18), 3.04 (H-23), 3.43 (H-3), 5.13 (brs, H-12), 7.23 (1H, m, H-2a), 7.32 (H-3a), 7.61 (−NH), 7.86 (2H, m, H-5a); APT-NMR (150 MHz, pyridine-d5): δ=13.1 (C-24), 15.9 (C-25), 17.3 (C-26), 23.9 (C-30), 26.0 (C-27), 33.3 (C-29), 37.7 (C-1), 41.2 (C-18), 64.8 (C-23), 70.7 (C-3), 112.5 (C-4a), 119.5 (C-2a), 121.9 (C-12), 123.7 (C5a), 128.7 (C-3a), 133.5 (C-2b), 144.2 (C-13), 152.4 (C-1b), 167.9 (C-1a), 179.1 (C-28); LC/MS (ESI-MS) m/z Calc for C37H53BrN2OS [M+Na]+: 694.11 (100) Found: 694.13.
Synthesis of (3β)-3-Hydroxy-23-(2-Anthrylamino) Olean-12-en-28-Oic Acid (2 f)
Column chromatography (dichloromethane/ethyl acetate (1/1)) gave pure 2 f as white solid (50 %); Rf=0.40 (DCM /EtOAc 1 : 1); m.p. 122.6–123.2 °C; IR KBr (cm−1): 3466, 2925, 2850, 2059, 1634, 1462, 1265, 1250, 761; 1H-NMR (600 MHz, DMSO-d6):=0.51 (s, H-24), 0.69 (s, H-26), 0.84 (s, H-30), 0.85 (s, H-25), 0.85 (s, H-29), 1.08 (s, H-27), 2.70 (dd, J=6.8, 13.4 Hz, H-18), 3.03-3.41 (H-23), 4.13 (m, H3), 5.13 (brs, H-12), 5.13 (brs, H-12), 7.04 (H-1a), 7.06 (H-3a), 7.66 (H-6a/H-7a), 7.69 (H-4a), 7.93 (H8a), 7.99 (H-5a), 8.07 (H-9a/H-10a);APT-NMR (150 MHz, DMSO-d6): 11.2 (C-24), 13.1 (C-25), 14.4 (C-26), 15.9 (C-27), 23.8 (C-30), 26.01 (C-29), 41.8 (C-18), 64.6 (C-23), 67.8 (C-5), 70.6 (C-3), 120.5 (C-9a), 121.9 (C-12), 123.0 (C-2b), 124.3 (C-4b), 127.7 (C-3a), 127.8 (C-6a/C-7a), 129.1 (C1a/C-8a), 132.0 (C-3b), 132.3 (C-4a), 137.3 (C-1b), 139.3 (C-2a), 144.3 (C-13), 179.3 (C-28); LC/MS (ESI-MS) m/z Calc for C44H57NO3 [M+2 K]+ : 683.84 Found: 683.87.
Biological Activity
In vitro cytotoxicity: MCF-7 (human metastatic breast adenocarcinoma), HeLa (human cervix adenocarcinoma), Jurkat (human lymphocytic cancer cell), K562 (human erythroleukemic cell) and L929 mouse fibroblast cells (healthy) were used in this study. MCF-7, HeLa, Jurkat and K562 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). Imatinib was purchased from Sigma-Aldrich Chemie GmbH (Steinheim, Deutschland).
HeLa, MCF-7, K562 and L929 cells were grown in DMEM, and Jurkat cell line was cultured in RPMI-1640 medium (Sigma-Aldrich, Germany). All cell lines were maintained in their logarithmic phase of growth before each experiment and subcultured when they reached 70–80 % confluency. The cells were grown in T-25 flasks as adherent monolayers in a %5 carbon dioxide (CO2) atmosphere at 37 °C using a culture medium, supplemented with %10 fetal bovine serum (FBS, Sigma-Aldrich, Germany) and antibiotics. Imatinib, a commercial classical anticancer drug, was used as a reference agent. The human cancer cells were harvested when they were confluent of 80 % and they were seeded into 96-well plates with 105 cell density of 106 cells/mL. Imatinib was dissolved in the culture medium with 1 % DMSO to give various concentrations (0.1 μM, 1 μM, 10 μM, and 100 μM, respectively). The resulted solutions were subsequently added to a set of wells. Control wells contained supplemented media with 1 % DMSO. The 96 well plates were incubated at 37 °C in a humidified atmosphere of 5 % CO2/95 % air for a further 72 h. The cytotoxic effects of compounds were measured using the 3-(4,5-dimethylthiazol-2-yl)-2,5-di-phenyltetrazolium bromide (MTT) assay. At the end of each incubation period, the MTT solution (10 μL, 5 mg/mL) was added to each well and the cultures were incubated further 48 h (for the time-dependent cytotoxic effects studies, the treatment time is 24 h, 48 h, 72 h, respectively) at 37 °C in a humidified atmosphere of 5 % CO2/95 % air. After 4 h of incubation, DMSO (100 μL) was added to dissolve the formazan crystals. The growth inhibition of cells was analyzed by measuring the absorbance of the drug-treated wells with respect to untreated ones at 560–620 nm using a plate reader (Biotek, Epoch, USA and Thermo Multiskan Spectrum, Waltham, MA). IC50 values (drug concentrations responsible for %50 cell growth inhibition) were calculated by GraphPad Prism 7 (GraphPad Software, San Diego, CA, USA). All the tests were repeated in at least three independent experiments; in a single experiment, each concentration was assayed in triplicate. All experiments were run in triplicate and the IC50 values were calculated from the results of the MTT assay. The results presented in tables as the drug concentrations (μM) that reduced the absorbance to 50 % of control values.
Detection of cell apoptosis: Apoptosis mechanism was evaluated by Hoechst 33342 & propidium staining (HOPI) showing necrotic, early and late apoptotic cells after tested compounds were cultured with cells in different concentrations.46 Initially, the cells were seeded in a 24 well plate at a density of 106 cells/well. After 24 h, the cells were treated with compounds. On the following day after treatments, the cells were harvested and washed with PBS, trypsinized, and centrifuged. Afterwards, 5 μL of the mixture of Hoechst 33342 and propidium iodide were added to the cells, mixed gently and incubated for 1 h at 37 °C in the dark. After incubation, 30 μL of cell solution was placed on a slide and was explored under a fluorescent microscope (Olympus BX51, Tokyo, Japan) and photographed. The number of living, apoptotic and necrotic cells were analyzed using ImageJ software (NIH, MD, USA).
Detection of immune pathway: Immune pathway was determinated by Fibroblast Growth Factor 1 (FGF-1), Interleukin 1 (IL-1), Interleukin 6 (IL-6), and Tumor Necrosis Factor Alpha (TNF-α) Elisa kits according to the instructions of the manufacturer.
Antimicrobial activity: The determination of antimicrobial activity of gypsogenin and six new gypsogenin derivatives were studied in vitro by the microdilution method determined by the international Clinical and Laboratory Standards Institute (CLSI) and EUCAST, formerly known as NCCLS (National Committee for Clinical Laboratory Standards).47, 48 The antimicrobial activity of these new gypsogenin derivatives were evaluated against different strains of Gram-negative bacteria (Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, Gram-positive bacteria (Stapylococcus aureus ATCC 25923, Bacillus subtilis ATCC 6633, and Entereococcus faecalis ATCC 29212), and yeast (Candida albicans ATCC 90028). The minimal inhibitory concentration (MIC) method, which was determined and standardized by CLSI, was used as a method. The strains were taken from the stock medium at −80 °C, bacterial strains were inoculated on Mueller-Hinton agar (MHA) plate media, and yeast strains were cultivated on Sabouraud dextrose agar (SDA) plate media and resuscitated by incubating at 37 °C for 18–20 h. Suspensions were prepared from fresh cultures with 0.9 % NaCl solution (saline) and the suspensions were adjusted to 0.5 McFarland 1–2×108 Colony Forming Unit (CFU)/mL with McFarland instrument (Den-1, Biosan). The suspensions were diluted by 1/100. 50 μL of Mueller-Hinton II broth (cation added) (MHIIB) for bacterial strains and RPMI-1640 (MOPS-buffered) medium for yeast strains were dispensed into the wells of sterile 96-well microplates. 50 μL of active ingredient solutions (DMSO/distilled water, 1/1) was added to the wells in the first row and serial dilutions were prepared by transferring them to the side wells. 50 μL of bacterial and fungal suspensions were added to the wells. The lowest concentrations that did not show growth after incubation for 16–24 h at 37 °C were considered MICs. Medium and growth controls were performed on each microplate. Streptomycin and ketoconazole were used as standard antimicrobial agents.
Molecular docking studies: The crystal structure of the fibroblast growth factor receptor-1 (FGFR-1) kinase domain was retrieved from the RSCB database (PDB ID: 5UR1).49 The PrepWizard module of Maestro was used for preparing the raw file for the docking analysis, whereas compounds were prepared with energy minimization using OPLS_2005 force field at physiological pH using the LigPrep module. Grid generation of Maestro was used to determine the docking grid for the subsequent docking procedures, which were carried out for all compounds by Glide/SP docking protocols.50-52
4 Supporting Information
Additional data that support the findings of this study are available in the supplementary material.
Acknowledgments
This work was supported by Scientific Research Projects of Ege University (17FEN064). We are grateful to Professor Dr. Ali Özmen and Research Associate Dr. Ömer Erdoğan for biological activity studies. We also would like to thank to Dr. Belgin Sever for molecular docking studies.
Conflict of interests
This work was supported by Scientific Research Projects of Ege University (17FEN064).We applied for a “National Patent” for this study. We received a positive response from the first stage.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.