Synthesis and Inhibitor Effect Novel Alkoxymethyl Derivatives of Dihetero Cycloalkanes on Carbonic Anhydrase and Acetylcholinesterase
Vagif Farzaliyev
Institute of Chemistry of Additives, Ministry of Science and Education of the Republic of Azerbaijan, 1029 Baku, Azerbaijan
Baku State University, Z. Khalilov Str. 23, AZ-1148 Baku, Azerbaijan
Search for more papers by this authorAdem Ertürk
Ataturk University, Faculty of Science, Department of Chemistry, 25240 Erzurum, Türkiye
Search for more papers by this authorMalahat Abbasova
Institute of Chemistry of Additives, Ministry of Science and Education of the Republic of Azerbaijan, 1029 Baku, Azerbaijan
Search for more papers by this authorOruj Nabiyev
Institute of Chemistry of Additives, Ministry of Science and Education of the Republic of Azerbaijan, 1029 Baku, Azerbaijan
Search for more papers by this authorCorresponding Author
Dr. Yeliz Demir
- [email protected]
- [email protected]
- +905363759547 | Fax: +904782117552
Ardahan University, Nihat Delibalta Göle Vocational High School, Department of Pharmacy Services, 75700 Ardahan, Türkiye
Search for more papers by this authorHatice Kızıltaş
Van Yüzüncü Yıl University, Van Vocational School of Health Services, 65080 Van, Türkiye
Contribution: Formal analysis (equal), Investigation (equal)
Search for more papers by this authorAfsun Sujayev
Institute of Chemistry of Additives, Ministry of Science and Education of the Republic of Azerbaijan, 1029 Baku, Azerbaijan
Search for more papers by this authorİlhami Gülçin
Ataturk University, Faculty of Science, Department of Chemistry, 25240 Erzurum, Türkiye
Search for more papers by this authorVagif Farzaliyev
Institute of Chemistry of Additives, Ministry of Science and Education of the Republic of Azerbaijan, 1029 Baku, Azerbaijan
Baku State University, Z. Khalilov Str. 23, AZ-1148 Baku, Azerbaijan
Search for more papers by this authorAdem Ertürk
Ataturk University, Faculty of Science, Department of Chemistry, 25240 Erzurum, Türkiye
Search for more papers by this authorMalahat Abbasova
Institute of Chemistry of Additives, Ministry of Science and Education of the Republic of Azerbaijan, 1029 Baku, Azerbaijan
Search for more papers by this authorOruj Nabiyev
Institute of Chemistry of Additives, Ministry of Science and Education of the Republic of Azerbaijan, 1029 Baku, Azerbaijan
Search for more papers by this authorCorresponding Author
Dr. Yeliz Demir
- [email protected]
- [email protected]
- +905363759547 | Fax: +904782117552
Ardahan University, Nihat Delibalta Göle Vocational High School, Department of Pharmacy Services, 75700 Ardahan, Türkiye
Search for more papers by this authorHatice Kızıltaş
Van Yüzüncü Yıl University, Van Vocational School of Health Services, 65080 Van, Türkiye
Contribution: Formal analysis (equal), Investigation (equal)
Search for more papers by this authorAfsun Sujayev
Institute of Chemistry of Additives, Ministry of Science and Education of the Republic of Azerbaijan, 1029 Baku, Azerbaijan
Search for more papers by this authorİlhami Gülçin
Ataturk University, Faculty of Science, Department of Chemistry, 25240 Erzurum, Türkiye
Search for more papers by this authorAbstract
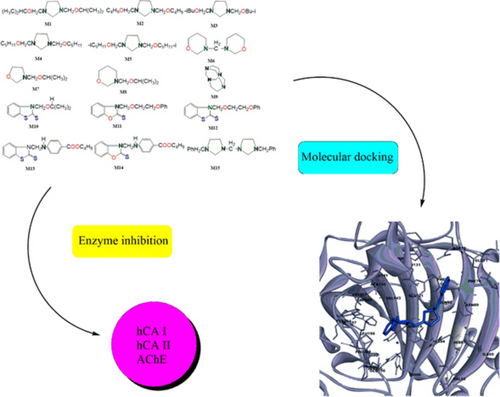
1,3-Diheterocycloalkanes derivatives are important starting materials in fine organic synthesis. These compounds can be widely used in various fields such as industry, medicine, biotechnology and chemical technology. The paper is focused on synthesis and study of alkoxymethyl derivatives of diheterocycloalkanes (M1–M15) and inhibition effect on carbonic anhydrase and acetylcholinesterase. The structures of compounds were confirmed by 1H and 13C NMR spectroscopy. Also, in this study alkoxymethyl derivatives of diheterocycloalkanes were assessed for their influence on various metabolic enzymes, including acetylcholinesterase (AChE) and human carbonic anhydrase isoenzymes (hCA I and hCA II). The results demonstrated that all these compounds exhibited potent inhibitory effects on all the target enzymes, surpassing the standard inhibitors, as evidenced by their IC50 and Ki values. The Ki values for the compounds concerning AChE, hCA I, and hCA II enzymes were in the ranges of 1.02±0.17–8.38±1.02, 15.30±3.15–58.14±5.17 and 24.05±3.70–312.94±27.24 nM, respectively.
Graphical Abstract
Conflict of interests
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| cbdv202400296-sup-0001-misc_information.pdf460.7 KB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1D. L. Rakhmankulov, V. V. Zorin, F. N. Latypova, S. S. Zlotsky, Chem. Heterocycl. Compd. 1982, 4, 435–449.
- 2V. M. Farzaliyev, M. T. Abbasova, G. B. Babaeva, Z. K. Soltanova, Az. Chem. Journal 2007, 2, 15–17.
- 3V. M. Farzaliyev, M. T. Abbasova, Z. K. Soltanova, G. B. Babaeva, N. P. Ladokhin, Az. Chem. Journal 2005, 2, 23–26.
- 4V. M. Farzaliyev, M. T. Abbasova, Z. K. Soltanova, L. R. Safarov, Russ. J. Org. Chem. 2022, 58, 643–649.
- 5V. M. Farzaliev, M. T. Abbasova, A. A. Ashurova, G. B. Babaeva, N. P. Ladokhina, Ya. M. Kerimova, Russ. J. Appl. Chem. 2009, 82, 928–930.
- 6V. M. Farzaliyev, M. T. Abbasova, N. P. Ladokhin, G. B. Babaeva, L. R. Safarov, Technologies of oil and gas 2014, 1, 36.
- 7L. R. Safarova, Chem. Probl. 2022, 4 (20), 66–373.
- 8R. H. Albrakaty, N. A. Wazzan, I. B. Obot, Int. J. Electrochem. Sci. 2018, 13, 3535–3554.
- 9D. R. Gataullina, D. R. Mogilevtseva, G. N. Nugumanova, S. V. Bukharov, R. G. Tagasheva, R. Ya. Deberdeev, Russ. J. Gen. Chem. 2017, 87, 1919–1923.
- 10İ. Gülçın, M. Abbasova, P. Taslimi, Z. Huyut, L. R. Safarova, A. Sujayev, V. Farzaliyev, Ş. Beydemır, S. Alwasel, C. T. Supuran, J. Enzyme Inhib. Med. Chem. 2017, 32(1), 1174–1182.
- 11D. Osmaniye, C. Türkeş, Y. Demir, Y. Özkay, Ş. Beydemir, Z. A. Kaplancıklı, Arch. Pharm. 2022, 355(8), e2200132.
- 12B. Yigit, M. Yigit, D. Barut Celepci, Y. Gök, A. Aktaş, M. Aygün, P. Taslimi, I. Gulcin, ChemistrySelect 2019, 3 (27), 7976–7982.
- 13A. Karimov, A. Orujova, P. Taslimi, N. Sadeghian, B. Mammadov, H. S. Karaman, V. Farzaliyev, A. Sujayev, R. Taş, S. Alwasel, I. Gulcin, Bioorg. Chem. 2020, 99, 103762.
- 14N. Oztaskin, S. Goksu, Y. Demir, A. Maras, İ. Gulcin, Molecules 2022, 27 (21), 7426.
- 15A. Aktas, D. Barut Celepci, Y. Gök, P. Taslimi, H. Akıncıoğlu, I. Gulcin, Crystals 2020, 10, 17.
10.3390/cryst10030171 Google Scholar
- 16L. Polat Köse, Z. Bingöl, R. Kaya, A. C. Gören, H. Akincioğlu, L. Durmaz, E. Koksal, S. Alwasel, I. Gulcin, Int. J. Food Prop. 2020, 23 (1), 878–893.
- 17N. Oztaskin, R. Kaya, A. Maraş, E. Sahin, I. Gulcin, S. Goksu, Bioorg. Chem. 2019, 87, 91–102.
- 18S. Bal, O. Demirci, B. Sen, T. Taskın Tok, P. Taslimi, A. Aktas, Y. Gok, M. Aygun, I. Gulcin, Appl. Organomet. Chem. 2021, 35 (9), e6312.
- 19C. Yamali, H. I. Gul, A. Ece, P. Taslimi, I. Gulcin, Chem. Biol. Drug Des. 2018, 91, 854–866.
- 20D. O. Ozgun, C. Yamali, H. I. Gul, P. Taslimi, I. Gulcin, T. Yanik, C. T. Supuran, J. Enzyme Inhib. Med. Chem. 2016, 31, 1498–1501.
- 21B. Özgeris, S. Göksu, L. Polat Köse, I. Gülçin, R. E. Salmas, S. Durdagi, F. Tümer, C. T. Supuran, Bioorg. Med. Chem. 2016, 24, 2318–2329.
- 22N. Lolak, S. Akocak, C. Türkeş, P. Taslimi, M. Işık, Ş. Beydemir, İ. Gülçin, M. Durgun, Bioorg. Chem. 2020, 100, 103897.
- 23G. Johnson, S. Moore, Curr. Pharm. Des. 2005, 12, 217–225.
- 24B. Turan, K. Şendil, E. Şengül, M. S. Gültekin, P. Taslimi, İ. Gulçin, C. T. Supuran, J. Enzyme Inhib. Med. Chem. 2016, 31, 79–88.
- 25S. F. Nelsen, R. J. Hintz, J. Am. Chem. Soc. 1972, 94, 7114.
- 26J. A. Verpoorte, S. Mehta, J. T. Edsall, J. Biol. Chem. 1967, 242, 4221–4229.
- 27C. Kakakhan, C. Türkeş, Ö. Güleç, Y. Demir, M. Arslan, G. Özkemahlı, Ş. Beydemir, Bioorg. Med. Chem. 2023, 77, 117111.
- 28A. Buza, C. Türkeş, M. Arslan, Y. Demir, B. Dincer, A. R. Nixha, Ş. Beydemir, Int. J. Biol. Macromol. 2023, 239, 124232.
- 29G. L. Ellman, K. D. Courtney, V. Andres, Biochem. Pharmacol. 1961, 7, 88–95.
- 30F. Turkan, A. Cetin, P. Taslimi, İ. Gulçin, Arch. Pharm. 2018, 351(10), e1800200.
- 31P. Taslimi, F. Türkan, D Güngördü Solğun, A. Aras, Y. Erden, H. U. Celebioglu, B. Tuzun, M. S. Agırbas, S. Gunay, I. Gulcin, J. Biomol. Struct. Dyn. 2022, 40(7), 2991–3002.
- 32Y. Gull, N. Rasool, M. Noreen, A. A. Altaf, S. G. Musharraf, M. Zubaır, F.-U.-H. Nasim, A. Yaqoob, V. DeFeo, M. Zia-Ul-Haq, Molecules 2016, 21(3), 266.
- 33A. A. Altaf, M. Hamayun, B. Lal, M. N. Tahir, A. A. Holder, A. Badshah, D. C. Crans, Dalton Trans. 2018, 47(34), 11769–11781.
- 34A. Malik, N. Rasool, I. Kanwal, M. A. Hashmı, A. F. Zahoor, G. Ahmad, A. A. Altaf, S. A. A. Shah, S. Sultan, Z. A. Zakaria, Processes 2020, 8(11), 1342.
- 35H. M. Berman, T. Battistuz, T. N. Bhat, W. F. Bluhm, P. E. Bourne, K. Burkhardt, Z. Feng, G. L. Gilliland, L. Iype, S. Jain, P. Fagan, J. Marvin, D. Padilla, V. Ravichandran, B. Schneider, N. Thanki, H. Weissig, J. D. Westbrook, C. Zardecki, Acta Crystallogr. Sect. D 2002, 58, 899–907.
- 36O. Trott, A. J. Olson, J. Comput. Chem. 2010, 31(2), 455–461.
- 37S. Salentin, S. Schreiber, V. J. Haupt, M. F. Adasme, M. Schroeder, Nucleic Acids Res. 2015, 43, 443–447.
- 38M. Tugrak, H. I. Gul, Y. Demir, S. Levent, I. Gulcin, Arch. Pharm. 2021, 354, e2000375.
- 39C. Yamali, H. İ. Gül, Y. Demir, C. Kazaz, İ. Gülçin, Turk. J. Chem. 2020, 44(4), 1058.
- 40M. S. Özaslan, R. Sağlamtaş, Y. Demir, Y. Genç, İ. Saraçoğlu, İ. Gülçin, Chem. Biodiversity 2022, 19 (8), e202200280.
- 41S. Bilginer, H. I. Gul, B. Anil, Y. Demir, I. Gulcin, Arch. Pharm. 2021, 354 (1), 2000243.
- 42S. Ayvaz, M. Çankaya, A. Atasever, A. Altuntas, J. Enzyme Inhib. Med. Chem. 2013, 28(2), 305–310.
- 43A. Altundas, B. Gül, M. Çankaya, A. Atasever, İ. Gülçin, J. Biochem. Mol. Toxicol. 2017, 31(12), e21998.