A New Insight Into The Huisgen Reaction: Heterogeneous Copper Catalyzed Azide-Alkyne Cycloaddition for the Synthesis of 1,4-Disubstituted Triazole (From 2018–2023)
Shivani Kasana
Department of Pharmaceutical Chemistry, ISF College of Pharmacy, Moga, 142001 Punjab, India
Search for more papers by this authorVaibhav Nigam
Department of Pharmaceutical Chemistry, ISF College of Pharmacy, Moga, 142001 Punjab, India
Search for more papers by this authorSurbhi Singh
Department of Pharmaceutical Chemistry, ISF College of Pharmacy, Moga, 142001 Punjab, India
Search for more papers by this authorBalak Das Kurmi
Department of Pharmaceutics, ISF College of Pharmacy, Moga, 142001 Punjab, India
Search for more papers by this authorCorresponding Author
Preeti Patel
Department of Pharmaceutical Chemistry, ISF College of Pharmacy, Moga, 142001 Punjab, India
Search for more papers by this authorShivani Kasana
Department of Pharmaceutical Chemistry, ISF College of Pharmacy, Moga, 142001 Punjab, India
Search for more papers by this authorVaibhav Nigam
Department of Pharmaceutical Chemistry, ISF College of Pharmacy, Moga, 142001 Punjab, India
Search for more papers by this authorSurbhi Singh
Department of Pharmaceutical Chemistry, ISF College of Pharmacy, Moga, 142001 Punjab, India
Search for more papers by this authorBalak Das Kurmi
Department of Pharmaceutics, ISF College of Pharmacy, Moga, 142001 Punjab, India
Search for more papers by this authorCorresponding Author
Preeti Patel
Department of Pharmaceutical Chemistry, ISF College of Pharmacy, Moga, 142001 Punjab, India
Search for more papers by this authorAbstract
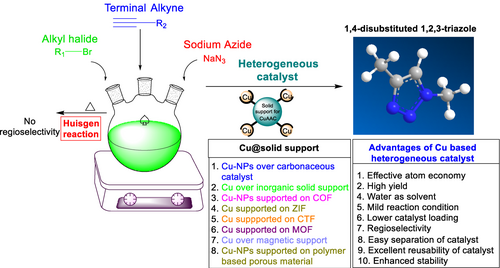
The Huisgen cycloaddition, often referred to as 1,3-Dipolar cycloaddition, is a well-established method for synthesizing 1,4-disubstituted triazoles. Originally conducted under thermal conditions [3+2] cycloaddition reactions were limited by temperature, prolonged reaction time, and regioselectivity. The introduction of copper catalyzed azide-alkyne cycloaddition (CuAAC) revitalized interest, giving rise to the concept of “click chemistry”. The CuAAC has emerged as a prominent method for producing 1,2,3-triazole with excellent yields and exceptional regioselectivity even in unfavorable conditions. Copper catalysts conventionally facilitate azide-alkyne cycloadditions, but challenges include instability and recycling issues. In recent years, there has been a growing demand for heterogeneous and porous catalysts in various chemical reactions. Chemists have been more interested in heterogenous catalysts as a result of the difficulties in separating homogenous catalysts from reaction products. These catalysts are favored for their abundant active sites, extensive surface area, easy separation from reaction mixtures, and the ability to be reused. Heterogeneous catalysts have garnered significant attention due to their broad industrial utility, characterized by cost-effectiveness, stability, resistance to thermal degradation, and ease of removal compared to their homogeneous counterparts. The present review covers recent advancements from year 2018 to 2023 in the field of click reactions for obtaining 1,2,3-triazoles through Cu catalyzed 1,3-dipolar azide-alkyne cycloaddition and the properties of the catalyst, reaction conditions such as solvent, temperature, reaction time, and the impact of different heterogeneous copper catalysts on product yield.
Graphical Abstract
Conflict of interests
The authors declare no conflict of interest.
References
- 1M. Breugst, H. U. Reissig, Angew. Chem. Int. Ed. 2020, 59, 12293–12307.
- 2I. I. Mangalagiu, Molecules 2023, 28, 5692.
- 3K. Hema, K. M. Sureshan, Acc. Chem. Res. 2019, 52, 3149–3163.
- 4P. Saini, G. Singh, G. Kaur, J. Singh, H. Singh, J. Mol. Catal. 2021, 504, 111432.
- 5M. M. Maphupha, A. Vidov, C. B. de Koning, D. Brady, Biocatal. Biotransform. 2022, 1–11.
- 6S. Boggala, V. Perupogu, S. Varimalla, K. Manda, V. Akula, J. Mol. Catal. 2022, 521, 112191.
- 7A. K. Agrahari, P. Bose, M. K. Jaiswal, S. Rajkhowa, A. S. Singh, S. Hotha, N. Mishra, V. K. Tiwari, Chem. Rev. 2021, 121, 7638–7956.
- 8V. V. Rostovtsev, L. G. Green, V. V. Fokin, K. B. Sharpless, Angew. Chem. 2002, 114, 2708–2711.
10.1002/1521-3757(20020715)114:14<2708::AID-ANGE2708>3.0.CO;2-0 Google Scholar
- 9C. W. Tornøe, C. Christensen, M. Meldal, J. Org. Chem. 2002, 67, 3057–3064.
- 10A. Pucci, G. Albano, M. Pollastrini, A. Lucci, M. Colalillo, F. Oliva, C. Evangelisti, M. Marelli, D. Santalucia, A. Mandoli, Catalysts 2020, 10, 434.
- 11H. Wei, Z. Liu, H. Zhu, J. He, J. Li, Polym. Eng. Sci. 2020, 60, 917–924.
- 12C. Wang, J. Yang, Y. Lu, Chin. J. Catal. 2023, 49, 8–15.
- 13A. F. Kareem, S. F. Abbas, H. A. Mubarak, H. H. Alwan, Mag. Al-Kufa Univ. biol. 2023, 15, 35–44.
- 14K. K. Tremblay-Parrado, C. García-Astrain, L. Avérous, Green Chem. 2021, 23, 4296–4327.
- 15Y. Yousfi, W. Benchouk, S. M. Mekelleche, Theor. Chem. Acc. 2021, 140, 4.
- 16A. Tripolszky, E. Tóth, P. T. Szabó, L. Hackler Jr, B. Kari, L. G. Puskás, E Bálint, Molecules 2020, 25, 2643.
- 17P. Phukan, R. Chetia, R. Boruah, S. Konwer, D. Sarma, Mater Adv 2021, 2, 6996–7006.
- 18S. K. Avula, A. Khan, S. A. Halim, Z. Al-Abri, M. U. Anwar, A. Al-Rawahi, R. Csuk, A. Al-Harrasi, Bioorg. Chem. 2019, 91,103182.
- 19B. Kodasi, S. D. Joshi, R. R. Kamble, R. S. Keri, P. K. Bayannavar, A. R. Nesaragi, S. Dixit, S. K. Vootla, T. V. Metre, Appl. Organomet. Chem. 2022, 36, e6664.
- 20T. V. Metre, B. Kodasi, P. K. Bayannavar, L. Bheemayya, V. B. Nadoni, S. R. Hoolageri, A. K. Shettar, S. D. Joshi, V. M. Kumbar, R. R. Kamble, Bioorg. Chem. 2023, 130, 106235.
- 21P. K. Bangalore, S. K. Vagolu, R. K. Bollikanda, D. K. Veeragoni, P. C. Choudante, S. Misra, D. Sriram, B. Sridhar, S. Kantevari, J. Nat. Prod. 2019, 83, 26–35.
- 22A. Rammohan, B. C. Venkatesh, N. M. Basha, G. V. Zyryanov, A. Nageswararao, Chem. Biol. Drug Des. 2023, 101, 1181–1203.
- 23İ. Şahin, F. B. Özgeriş, M. Köse, E. Bakan, F. Tümer, J. Mol. Struct. 2021, 1232, 130042.
- 24M. M. Matin, P. Matin, M. R. Rahman, T. Ben Hadda, F. A. Almalki, S. Mahmud, M. M. Ghoneim, M. Alruwaily, S. Alshehri, Front. Mol. Biosci. 2022, 9, 864286.
- 25T. R. Deshmukh, S. P. Khare, V. S. Krishna, D. Sriram, J. N. Sangshetti, O. Bhusnure, V. M. Khedkar, B. Shingate, J. Heterocycl. Chem. 2019, 56, 2144–2162.
- 26M. Marzi, M. Farjam, Z. Kazeminejad, A. Shiroudi, A. Kouhpayeh, E. Zarenezhad, J. Chem. 2022, 1–50.
10.1155/2022/7884316 Google Scholar
- 27Z. Xu, Eur. J. Med. Chem. 2020, 206, 112686.
- 28H. C. Upadhyay, Curr. Top. Med. Chem. 2021, 21, 737–752.
- 29D. Ashok, M. R. Reddy, R. Dharavath, K. Ramakrishna, N. Nagaraju, M. Sarasija, J. Chem. Sci. 2020, 132, 1–9.
- 30Y. Deswal, S. Asija, D. Kumar, Res. Chem. Intermed. 2019, 389, 82–124. “.
- 31M. C. Joseph, A. J. Swarts, S. F. Mapolie, Coord. Chem. Rev. 2022, 456, 215317.
- 32S. V. Amosova, A. V. Martynov, V. A. Potapov, J. Organomet. Chem. 2022, 977, 122442.
- 33B. R. Nemallapudi, D. R. Guda, N. Ummadi, B. Avula, G. V. Zyryanov, C. S. Reddy, S. Gundala, Polycycl Aromat Comp. 2022, 42, 3874–3892.
- 34Z. G. Wu, X. J. Liao, L. Yuan, Y. Wang, Y. X. Zheng, J. L. Zuo, Y. Pan, Chem. Eur. J. 2020, 26, 5694–5700.
- 35S. Gupta, C. Ameta, R. Ameta, P. B. Punjabi, Green Sustainable Process for Chemical and Environmental Engineering and Science 2020, 13–48.
- 36Sethiya, A. N. Sahiba, S. Agarwal, Current Topics in Chirality-From Chemistry to Biology, 2021, IntechOpen.
- 37M. B. Hammouda, I. Ahmad, A. Hamdi, A. Dbeibia, H. Patel, N. Bouali, W. S. Hamadou, K. Hosni, S. Ghannay, F. Alminderej, E. Noumi, Arab. J. Chem. 2022, 15, 104226.
- 38T. Liang, X. Sun, W. Li, G. Hou, F. Gao, Front. Pharmacol. 2021, 12, 661173.
- 39M. Bakherad, R. Doosti, Z. Qasemifar, J. Appl. Chem. Res. 2020, 14, 8–20.
- 40M. Bakherad, A. Keivanloo, A. Abdia, S. Kamrani Tamardasha, J. Appl. Chem. Res. 2022, 16, 36–47.
- 41T. Abiraman, K. Rajavelu, P. Rajakumar, S. Balasubramanian, ACS Omega 2020, 5, 7815–7822.
- 42S. López, J. M. García-Vargas, M. T. García, J. F. Rodríguez, I. Gracia, M. J. Ramos, Catalysts 2022, 12, 194.
- 43B. Li, R. Hu, A. Qin, B. Z. Tang, Polym. Chem. 2020, 11, 2006–14.
- 44P. Hoffmann, C. Lherbet, I. Fabing, M. C. Barthélémy, Y. Borjon-Piron, C. Laurent, A. Vigroux . RSC Adv. 2022, 12, 26825–26833.
- 45S. Sethi, N. C. Jana, S. Behera, R. R. Behera, B. Bagh, ACS Omega 2022, 8, 868–878.
- 46X. Cai, J. Nie, C. Lu, F. Wang, C. Ma, G. Yang, Z. Chen, Y. Zhang, Microporous Mesoporous Mater. 2021, 310, 110671.
- 47F. Xiang, B. Li, P. Zhao, J. Tan, Y. Yu, S. Zhang, Adv. Synth. Catal. 2019, 361, 5057–5062.
- 48C. Z. Tao, X. Cui, J. Li, A. X. Liu, L. Liu, Q. X. Guo, Tetrahedron Lett. 2007, 48, 3525–3529.
- 49E. Haldón, M. C. Nicasio, P. J. Pérez, Org. Biomol. Chem. 2015, 13, 9528–50.
- 50E. Leyva, I. R. Rodríguez-Gutiérrez, E. Moctezuma, S. Noriega, Curr. Org. Chem. 2022 26, 2098–121.
- 51N. Touj, A. Chakchouk-Mtibaa, L. Mansour, A. H. Harrath, J. H. Al-Tamimi, I. Özdemir, L. Mellouli, S. E. Yaşar, N. Hamdi, J. Organomet. Chem. 2017, 853, 49–63.
- 52J. B. Binder, R. T. Raines, Org. Lett. 2008, 10, 4279–4282.
- 53Q. Wang, T. R. Chan, R. Hilgraf, V. V. Fokin, K. B. Sharpless, J. Am. Chem. Soc. 2003, 125, 3192–3193.
- 54N. Aflak, H. Ben El Ayouchia, L. Bahsis, H. Anane, M. Julve, S. E. Stiriba, Int. J. Mol. Sci. 2022, 23, 2383.
- 55P. Ren, Q. Li, T. Song, Z. Wang, K. Motokura, Y. Yang, ChemCatChem 2021, 13, 3960–3966.
- 56H. Rajabi-Moghaddam, M. Naimi-Jamal, M. Tajbakhsh, Sci. Rep. 2021, 11, 2073.
- 57R. S. Gomes, G. A. Jardim, R. L. de Carvalho, M. H. Araujo, E. N. da Silva Júnior, Tetrahedron 2019, 75, 3697–3712.
- 58M. Ahlquist, V. V. Fokin, Organometallics 2007, 26, 4389–4391.
- 59B. T. Worrell, J. A. Malik, V. V. Fokin, Science 2013, 340, 457–460.
- 60H. M. Pineda-Castañeda, Z. J. Rivera-Monroy, M. Maldonado, ACS Omega 2023, 8, 3650–3666.
- 61Z. Zhang, P. Song, J. Zhou, Y. Chen, B. Lin, Y. Li, Ind. Eng. Chem. Res. 2016, 55, 12301–12308.
- 62A. Z. Ahmady, F. Heidarizadeh, M. Keshavarz, Synth. Commun. 2013, 43, 2100–2109.
- 63S. J. Kwak, U. S. Shin, S. H. Kim, Chem. Sci. 2023, 135, 10.
- 64A. G. Samuel, S. Subramanian, V. Vijendran, J. Bhagavathsingh, Front. Chem. 2022, 9, 754734.
- 65M. Eftekhar, F. Raoufi, Polycyclic Aromat. Compd. 2022, 42, 4780–4792.
- 66N. Buğday, S. Yaşar, S. Altin, O. Duygulu, Y. Önal, Appl. Organomet. Chem. 2023, 37, 7017.
10.1002/aoc.7017 Google Scholar
- 67P. Kalita, P. P. Sarma, P. Dutta, U. K. Gautam, P. K. Baruah, Polycyclic Aromat. Compd. 2023, 1–16.
- 68R. Eisavi, F. Ahmadi, Sci. Rep. 2022, 12, 11939.
- 69F. Salehzadeh, M. Esmkhani, M. Zallaghi, S. Javanshir, M. G. Dekamin, Sci. Rep. 2023, 13, 8675.
- 70M. Anvari, N. Shadjou, Monatsh. Chem. 2021, 152, 1277–1284.
- 71A. Pawar, S. Gajare, A. Jagdale, S. Patil, W. Chandane, G. Rashinkar, S. Patil, Catal. Lett. 2022, 152, 1854–1868.
- 72F. Godarzbod, Z. Mirjafary, H. Saeidian, M. Rouhani, Appl. Organomet. Chem. 2021, 35, 6132.
- 73A. Yadav, P. Patil, N. Birajdar, A. Gophane, K. C. Gunturu, S. Hangirgekar, S. Sankpal, Appl. Organomet. Chem. 2023, 37, 7181.
- 74C. Pathak, G. Bora, Chem. Pap. 2022, 76, 4749–4761.
- 75M. Daraie, D. Bagheri, M. Malmir, M. M. Heravi, Sci. Rep. 2021, 11, 23658.
- 76M. Torabi, M. A. Zolfigol, M. Yarie, Arab. J. Chem. 2023, 105090.
10.1016/j.arabjc.2023.105090 Google Scholar
- 77I. Romero-Muñiz, P. Albacete, A. E. Platero-Prats, F. Zamora, ACS Appl. Mater. Interfaces. 2021, 13, 54106–54112.
- 78A. P. Anbar, S. R. Delcheh, P. M.. Heynderickx, S. Chaemcheun, S. Zhuiykov, F. Verpoort, Catalyst 2023, 13, 1003.
10.3390/catal13061003 Google Scholar
- 79E. Afzali, Z. Mirjafary, A. Akbarzadeh, H. Saeidian, J. Inorg. Organomet. Polym. Mater. 2023, 1–11.
- 80G. Vilé, J. Liu, Z. Zhang, React. Chem. Eng. 2021, 6, 1878–1883.
- 81S. Bhagat, S. Dani, A. Verma, R. Dharavath, U. R. Pratap, J. Mol. Struct. 2023, 1284, 135350.
- 82Z. Wang, X. Zhou, S. Gong, J. Xie, Nanomaterials 2022, 12, 1070.
- 83I. Yamane, K. Sato, R. Otomo, T. Yanase, A. Miura, T. Nagahama, Y. Kamiya, T. Shimada, Nanomaterials 2021, 11, 1040.
- 84E. Arefi, A. Khojastehnezhad, A. Shiri, Sci. Rep. 2021, 11, 20514.
- 85S. S. Dehkordi, A. A. Jafari, J. Albadi, H. A. Samimi, Mol. Diversity 2023. 27, 177–192.
- 86A. R. Sardarian, F. Mohammadi, M. Esmaeilpour, Res. Chem. Intermed. 2019, 45, 1437–1456.
- 87H. K. Siuki, P. G. Kargar, G. Bagherzade, Front. Chem. 2021, 9, 1–19.
- 88B. J. Sarkar, M. Kundu, B Mondal, S. Mukherjee, A. Bandyopadhyay, U. K. Roy, J. Mol. Struct. 2023, 1274, 134493.
- 89F. Laffafchi, M. Tajbakhsh, Y. Sarrafi, B. Maleki, M. Ghani, Polycyclic Aromat. Compd. 2023, 43, 3240–3256.
- 90N. Khaleghi, Z. Mojtabapour, Z. Rashvandi, A. Mohammadi, M. Forouzandeh-Malati, F. Ganjali, S. Zarei-Shokat, A. Kashtiaray, R. Taheri-Ledari, A. Maleki, Nanoscale Adv. 2023, 5, 4911–4924.
- 91N. Aflak, E. M. El Mouchtari, H. Ben El Ayouchia, H. Anane, S. Rafqah, M. Julve, S. E. Stiriba, Catalysts 2022, 12, 1244.
- 92T. Goswami, S. Naithani, A. Kumar, S. Kumar, Colloids Surf. 2023, 662, 130982.
- 93M. Khoshnevis, H. Eshghi, Appl. Organomet. Chem. 2021, 35, e6281.
- 94S. Sampath, M. Vadivelu, A. A. Raheem, R. Indirajith, K. Parthasarathy, K. Karthikeyan, C. Praveen, Ind. amp. Eng.Chem. Res. 2022, 61, 9552–9566.
- 95T. Krishnaveni, K. Kadirvelu, M. Kaveri, MSEB 2022, 278, 115618.
- 96K. Barman, P. Dutta, D. Chowdhury, P. K. Baruah, BioNanoScience 2021, 11, 189–199.
- 97S. E. Stiriba, L. Bahsis, E. Benhadria, K. Oudghiri, M. Taourirte, M. Julve, Int. J. Mol. Sci. 2023, 24, 9301.
- 98P. Gupta, P. Kumar, B. Syal, T. Shamim, Res. Chem. Intermed. 2022, 48, 4601–4615.
- 99M. Kumar, K. Pakshirajan, New J. Chem. 2022, 46, 13953–13962.
- 100F. Jafarzadeh, Z. Dolatkhah, S. Molaei, S. Javanshir, Arab. J. Chem. 2022, 15, 103838.
- 101G. Vilé, G. Di Liberto, S. Tosoni, A. Sivo, V. Ruta, M. Nachtegaal, A. H. Clark, S. Agnoli, Y. Zou, A. Savateev, M. Antonietti, ACS Catal. 2022, 12, 2947–2958.
- 102D. S. de Sá, E. J. da Rocha Rodrigues, N. M. Suguihiro, A. G. da Veiga, S. Paciornik, A. Massi, O. Ginoble Pandoli, Catal. Lett. 2022, 152, 3558–3575.
- 103M. Zhang, J. Xu, T. Zhang, Y. Li, Res. Chem. Intermed. 2021, 47, 3883–3898.
- 104M. Aier, F. R. Gayen, A. Puzari, Sci. Rep. 2022, 12, 14613.
- 105M. Vafaeezadeh, J. Schaumlöffel, A. Lösch, A. De Cuyper, W. R. Thiel, ACS Appl. Mater. Interfaces 2021, 13, 33091–33101.
- 106H. Mirzaei, H. Eshghi, S. M. Seyedi, Appl. Organomet. Chem. 2021, 35, e6019.
- 107E. Afzali, Z. Mirjafary, A. Akbarzadeh, H. Saeidian, Inorg. Chem. Commun. 2021, 132, 108858.
- 108M. Darroudi, H. Rouh, M. Hasanzadeh, N. Shadjou, Heliyon 2021, 7.
- 109S. Mirshafiee, A. Salamatmanesh, A. Heydari, Appl. Organomet. Chem. 2021, 35, e6255.
- 110N. Sharma, M. Gupta, B. Chowhan, A. Frontera, J. Mol. Struct. 2021, 1224, 129029.