Synthesis, Characterization and Evaluation of the Antimicrobial and Herbicidal Activities of Some Transition Metal Ions Complexes with the Tranexamic Acid
Amira A. Mohamed
Department of Basic Science, Zagazig Higher Institute of Engineering and Technology, Zagazig, 44519 Egypt
Contribution: Conceptualization (lead), Data curation (equal), Formal analysis (supporting), Writing - original draft (lead), Writing - review & editing (supporting)
Search for more papers by this authorSadeek A. Sadeek
Department of Chemistry, Faculty of Science, Zagazig University, Zagazig, 44519 Egypt
Search for more papers by this authorNihad G. Rashid
Ministry of Education, Babylon, 51001 Iraq
Contribution: Formal analysis (equal), Investigation (supporting), Software (equal)
Search for more papers by this authorHazem S. Elshafie
School of Agricultural, Forestry, Food and Environmental Sciences, University of Basilicata, Viale dell'Ateneo, Lucano 10, Potenza, 85100 Italy
Contribution: Conceptualization (equal), Formal analysis (equal), Methodology (equal), Writing - original draft (equal), Writing - review & editing (lead)
Search for more papers by this authorCorresponding Author
Ippolito Camele
- [email protected]
- +39-0971-205544 | Fax: +39-0971-205503
School of Agricultural, Forestry, Food and Environmental Sciences, University of Basilicata, Viale dell'Ateneo, Lucano 10, Potenza, 85100 Italy
Contribution: Conceptualization (equal), Investigation (supporting), Writing - original draft (equal), Writing - review & editing (lead)
Search for more papers by this authorAmira A. Mohamed
Department of Basic Science, Zagazig Higher Institute of Engineering and Technology, Zagazig, 44519 Egypt
Contribution: Conceptualization (lead), Data curation (equal), Formal analysis (supporting), Writing - original draft (lead), Writing - review & editing (supporting)
Search for more papers by this authorSadeek A. Sadeek
Department of Chemistry, Faculty of Science, Zagazig University, Zagazig, 44519 Egypt
Search for more papers by this authorNihad G. Rashid
Ministry of Education, Babylon, 51001 Iraq
Contribution: Formal analysis (equal), Investigation (supporting), Software (equal)
Search for more papers by this authorHazem S. Elshafie
School of Agricultural, Forestry, Food and Environmental Sciences, University of Basilicata, Viale dell'Ateneo, Lucano 10, Potenza, 85100 Italy
Contribution: Conceptualization (equal), Formal analysis (equal), Methodology (equal), Writing - original draft (equal), Writing - review & editing (lead)
Search for more papers by this authorCorresponding Author
Ippolito Camele
- [email protected]
- +39-0971-205544 | Fax: +39-0971-205503
School of Agricultural, Forestry, Food and Environmental Sciences, University of Basilicata, Viale dell'Ateneo, Lucano 10, Potenza, 85100 Italy
Contribution: Conceptualization (equal), Investigation (supporting), Writing - original draft (equal), Writing - review & editing (lead)
Search for more papers by this authorAbstract
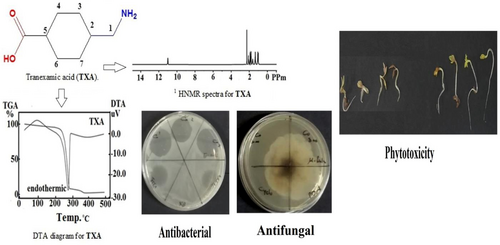
New tranexamic acid (TXA) complexes of ferric(III), cobalt(II), nickel(II), copper(II) and zirconium(IV) were synthesized and characterized by elemental analysis (CHN), conductimetric (Λ), magnetic susceptibility investigations (μeff), Fourier transform infrared (FT-IR), proton nuclear magnetic resonance (1H-NMR), ultraviolet visible (UV-vis.), optical band gap energy (Eg) and thermal studies (TG/DTG and DTA). TXA complexes were established in 1 : 2 (metal: ligand) stoichiometric ratio according to CHN data. Based on FT-IR and 1H-NMR data the disappeared of the carboxylic proton supported the deprotonating of TXA and linked to metal ions via the carboxylate group‘s oxygen atom as a bidentate ligand. UV-visible spectra and magnetic moment demonstrated that all chelates have geometric octahedral structures. Eg values indicated that our complexes are more electro conductive. DTA revealed presence of water molecules in inner and outer spheres of the complexes. DTA results showed that endothermic and exothermic peaks were identified in the degradation mechanisms. The ligand and metal complexes were investigated for their antimicrobial and herbicidal efficacy. The Co(II) and Ni(II) complexes showed antimicrobial activity against some tested species. The obtained results showed a promising herbicidal effect of TXA ligand and its metal complexes particularly copper and zirconium against the three tested plants.
Graphical Abstract
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| cbdv202301970-sup-0001-misc_information.pdf1 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1M. Hanif, Z. H. Chohan, Spectrochim. Acta Part A 2013, 104, 468–476.
- 2B. Lippert, Coord. Chem. Rev. 2000, 487, 200–202.
- 3N. Goswami, D. M. Eichhorn, Inorg. Chim. Acta. 2000, 303, 271–276.
- 4F. Dimiza, A. N. Papadopoulos, V. Tangoulis, V. Psycharis, C.P Raptopoulou, D. P, Kessissoglou, G. Psomas, J. Inorg. Biochem. 2012, 107, 54–64.
- 5E. Ortmann, M. W. Besser, A. A. Klein, Br. J. Anaesth. 2013, 111, 549–563.
- 6M. A. Longo, B. T. Cavalheiro, G. R. de Oliveira Filho, J. Clin. Anesth. 2018, 48, 32–38.
- 7Z. Dai, H. Chu, S. Wang, Y. Liang, J. Clin. Anesth. 2018, 44, 23–31.
- 8D. A. Henry, P. A. Carless, A. J. Moxey, D. O′Connell, B. J. Stokes, D. A. Fergusson, K. Ker, Cochrane Database Syst. Rev. 2011, 19.
- 9R. Karaman, H. Ghareeb, K. K. Dajani, L. Scrano, H. Hallak, H. H. Abu-Lafi, G. Mecca, S. A. Bufo, J. Comput.-Aided Mol. Des. 2013, 27, 615–635.
- 10A. S. Lukes, P. A. Kouides, K. A. Moore, Women′s Health 2011, 7(2), 151–158.
- 11A. S. Lukes, K. A. Moore, K. N. Muse, J. K. Gersten, B. R. Hecht, M. Edlund, H. E. Richter, S. E. Eder, G. R. Attia, D. L. Patrick, A. Rubin, G. A. Shangold, Obstet. Gynecol. 2010, 116(4), 865–875.
- 12E′. Ortmann, M. W. Besser, A. A. Klein, Br. J. Anaesth. 2013, 111, 549–563.
- 13J. D. Jennings, M. K. Solarz, C. Haydel, Orthop. Clin. North Am. 2016, 47, 137–143.
- 14K. Ker, D. Prieto-Merino, I. Roberts, Br. J. Surg. 2013, 100, 1271–1279.
- 15L. D. Pacheco, G. R. Saade, M. M. Costantine, S. L. Clark, G. D. Hankins, Am. J. Obstet. Gynecol. 2016, 214, 340–344.
- 16S. Sigaut, B. Tremey, A. Ouattara, R. Couturier, C. Taberlet, S. Grassin-Delyle, J. F. Dreyfus, S. Schlumberger, M. Fischler, Anesthesiology 2014, 120, 590–600.
- 17P. S. Myles, J. A. Smith, A. Forbes, B. Silbert, M. Jayarajah, T. Painter, D. J. Cooper, S. Marasco, J. McNeil, J. S. Bussieres, S. McGuinness, K. Byrne, M. T. Chan, G. Landoni, S. Wallace, N. Engl. J. Med. 2017, 376, 136–148.
- 18I. Roberts, H. Shakur, A. Afolabi, K. Brohi, T. Coats, Y. Dewan, S. Gando, G. Guyatt, B. J. Hunt, C. Morales, P. D. Perel, Prieto-Merino, T. Woolley, Lancet. 2011, 377, 1101–1096.
10.1016/S0140-6736(11)60317-6 Google Scholar
- 19A. Turki, W. Michael, Int. J. Neurol. 2011, 2011, 203579. doi:10.4061/2011/203579.
- 20W. Sufan, S. Hangyan, W. Hua, Y. Sheng, G. Jincai, S. Yi, P. Lei, Aesthet Plast. Surg. 2012, 36, 964–970.
- 21A. A. Helaly, A. A. El-Bindary, S. A. Elsayed, Int. Jo. Liq. 2023, 389, 122831.
- 22M. A. El-Bindary, A. A. El-Bindary, Appl. Organomet. Chem. 2022, 36, e6576.
- 23P. W. Hsieh, W. Y. Chen, A. Aljuffali, C. C. Chen, J. Y. Fang, Curr. Med. Chem. 2013, 20, 4080–4092.
- 24J. Song, W. Duan, Y. Chen, X. Liu, Chinese J. Struc. Chem. 2022, 41, 2205037–2205047.
- 25W. J. Geary, Coord. Chem. Rev. 1971, 7, 81–122.
- 26G. N. Rezk, O. A. El-Gammal, S. H. Alrefaee, I. Althagafi, A. A. El-Bindary, M. A. El-Bindary, Heliyon 2023, 9, e21015.
- 27O. A. El-Gammal, A. A. El-Bindary, F. Sh. Mohamed, G. N. Rezk, M. A. El-Bindary, Int. J. Liq. 2022, 346, 117850.
- 28S. M. Abd-El-Hamid, A. S. Sadeek, N. B. Sadek, M. A. Sabry, M. S. El-Gedamy, Int. J. Liq. In Press 2024.
- 29G. G. Mohamed, H. F. Abd El-Halim, M. M. I. El-Dessouky, W. H. Mahmoud, J. Mol. Struct. 2011, 999, 29–38.
- 30L. M. M. Vieira, M. V. de Almeida, M. C. S. Lourenço, F. A. F. M. Bezerra, A. P. S. Fontes, Eur. J. Med. Chem. 2009, 44, 4107–4111.
- 31M. M. Moawad, W. G. Hanna, J. Coord. Chem. 2002, 55, 439–457.
- 32H. S. Elshafie, S. A. Sadeek, I. Camele, A. A. Mohamed, Int. J. Mol. Sci. 2022, 23, 2110.
- 33S. Kumar, A. Rai, S. B. Rai, D. K. Rai, A. N. Singh, V. B. Singh, J. Mol. Struct. 2006, 791, 23–29.
- 34K. Nakamoto, Infrared Spectra of Inorganic and Coordination Compounds, Fourth ed., Wiely and Sons, New York, 1996.
- 35W. M. I. Hassan, M. A. Badawy, G. G. Mohamed, H. Moustafa, S. Elramly, Spectrochim. Acta Part A 2013, 111, 169–177.
- 36T. A. Yousef, G. M. Abu El-Reash, O. A. El-Gammal, R. A. Bedier, J. Mol. Str. 2013, 1035, 307–317.
- 37A. Tarushi, G. Psomas, C. P. Raptopoulou, V. Psycharis, D. P. Kessissoglou, Polyhedron. 2009, 28, 3272–3278.
- 38M. A. El-Ghamry, A. A. Saleh, S. M. E. Khalil, A. A. Mohammed, Spectrochim. Acta Part A 2013, 110, 205–216.
- 39A. Tarushi, C. P. Raptopoulou, V. Psycharis, A. Terzis, G. Psomas, D. P. Kessissoglou, Bioorg. Med. Chem. 2010, 18, 2678–2685.
- 40I. Turel, Coord. Chem. Rev. 2002, 232, 27–47.
- 41G. G. Mohamed, N. E. A. El-Gamel, F. N. Nour El-Dien, React. Inorg. Met. Org. Chem. 2001, 31, 347–358.
- 42D. R. Zhu, Y. Song, Y. Xu, Y. Zhang, S. S. S. Raj, H. K. Fun, X. Z. You, Polyhedron 2000, 19, 2019–2025.
- 43H. F. Abd El-halim, M. M. Omar, G. G. Mohamed, Spectrochim. Acta Part A 2011, 78, 36–44.
- 44S. Yadav, R. V. Singh, Spectrochim. Acta Part A 2011, 78, 298–306.
- 45B. Macias, M. Martinez, A. Sanchez, A. A. Dominguez-Gil, Int. J. Pharm. 1994, 106, 229–235.
- 46N. Mondal, D. K. Dey, S. Mitra, K. M. Abdul Malik, Polyhedron 2000, 19, 2707–2711.
- 47T. A. Mohamed, I. A. Shaaban, R. S. Farag, W. M. Zoghaib, M. S. Afifi, Spectrochim. Acta Part A 2015, 135, 417–427.
- 48G. G. Mohamed, E. M. Zayed, A. M. Hindy, Spectrochim. Acta Part A 2015, 145, 76–84.
- 49G. G. Mohamed, N. E. A. El-Gamel, F. Teixidor, Polyhedron 2001, 20, 2689–2696.
- 50M. M. Rashad, A. M. Hassan, A. M. Nassar, N. M. Ibrahim, A. Mourtada, Appl. Phys. A 2013, 117, 877.
- 51J. Tauc, J. Mater, Res. Bull. 1968, 3, 37.
- 52F. Karipcin, B. Dede, Y. Caglar, D. Hur, S. Ilican, M. Caglar, Y. Sahin, Opt. Commun. 2007, 272, 131.
- 53S. K. Sengupta, O. P. Pandey, B. K. Srivastava, V. Sharma, Transition Met. Chem. 1998, 23, 349.
- 54N. Turan, B. Gündüz, H. Körkoca, R. Adigüzel, N. Çolak, K. Buldurun, J. Mex. Chem. Soc. 2014, 58, 65.
- 55T. S. Lobana, A. Sánchez, J. S. Casas, J. Chem. Soc. 1997, 22, 4289–4299.
- 56P. Drevensêk, J. Kosmrlj, G. Giester, T. Skauge, E. Sletten, K. Sepcìc, I. Turel, J. Inorg. Biochem. 2006, 100, 1755–1763.
- 57S. A. Sadeek, W. H. El-Shwiniy, W. A. Zordok, A. M. El-Didamony, Spectrochim. Acta Part A 2011, 78, 854–867.
- 58T. Stringer, P. Chellan, B. Therrien, N. D. T. Shunmoogam-Gounden Hendricks, G. S. Smith, Polyhedron 2009, 28, 2839–2846.
- 59E. K. Efthimiadou, Y. Sanakis, N. Katsaros, A. Karaliota, G. Psomas, Polyhedron 2007, 26, 1148.
- 60S. S. Alias, A. B. Ismail, A. A. Mohamad, J. Alloys Compd. 2010, 499, 231–237.
- 61E. K. Efthimiadou, N. Katsaros, A. Karaliota, G. Psomas, Bioorg. Med. Chem. Lett. 2007, 17, 1238.
- 62A. W. Coats, J. P. Redfern, Nature 1964, 201, 68–69.
- 63H. H. Horowitz, G. Metzger, Traces. Anal. Chem. 1963, 35, 1464–1468.
- 64O. A. El Gammal, Inorg. Chim. Acta. 2015, 435, 73–81.
- 65S. H. Sakr, H. S. Elshafie, I. Camele, S. A. Sadeek, Molecules. 2018, 23, 1182.
- 66N. H. Patel, H. M. Parekh, M. N. Patel, Pharm. Chem. J. 2007, 1, 78–81.
10.1007/s11094-007-0017-2 Google Scholar
- 67F. I. Abouzayed, S. A. Abouel-Enein, A. M. Hammad, ACS Omega. 2021, 15, 27737–27754.
10.1021/acsomega.1c02989 Google Scholar
- 68Z. H. Chohan, K. M. Khan, C. T. Supuran, Appl. Organomet. Chem. 2004, 18, 305–310.
- 69H. S. Elshafie, L. De Martino, C. Formisano, L. Caputo, V. De Feo, I. Camele, Plants 2023, 12, 1869.
- 70H. S. Elshafie, I. Camele, R. Racioppi, L. Scrano, N. S. Iacobellis, S. A. Bufo, Int. J. Mol. Sci. 2012, 13, 16291–16302.
- 71F. Ceglie, H. S. Elshafie, V. Verrastro, F. Tittarelli, J. Compost Sci. Util. 2011, 19, 293–300.Manuscript received: December 12, 2023Version of record online: April 29, 2024