Biological Activity and Phytochemical Analysis of Dicranum scoparium against the Bacterial Disease for Honey Bee
Corresponding Author
Şengül Alpay Karaoğlu
Department of Biology, Faculty of Arts and Sciences, Recep Tayyip Erdoğan University, Rize, Turkey
Search for more papers by this authorNurettin Yayli
Faculty of Pharmacy, Karadeniz Technical University, Trabzon, Turkey
Search for more papers by this authorİshak Erik
Faculty of Pharmacy, Karadeniz Technical University, Trabzon, Turkey
Search for more papers by this authorBüşra Korkmaz
Faculty of Pharmacy, Karadeniz Technical University, Trabzon, Turkey
Search for more papers by this authorRahşan Akpinar
Laboratory of Bee Diseases, Veterinary Control Institute, Samsun, Turkey
Search for more papers by this authorArif Bozdeveci
Department of Biology, Faculty of Arts and Sciences, Recep Tayyip Erdoğan University, Rize, Turkey
Search for more papers by this authorŞeyma Suyabatmaz
Department of Biology, Faculty of Arts and Sciences, Recep Tayyip Erdoğan University, Rize, Turkey
Search for more papers by this authorNevzat Batan
Maçka Vocational School, Karadeniz Technical University, Trabzon, Turkey
Search for more papers by this authorAydın Yeşilyurt
Tonya Vocational School, Trabzon University, Trabzon, Turkey
Search for more papers by this authorSelma Kaya
Laboratory of Bee Diseases, Veterinary Control Institute, Samsun, Turkey
Search for more papers by this authorCevat Nisbet
Department of Biochemistry, Faculty of Veterinary Medicine, Ondokuz Mayıs University, Samsun, Turkey
Search for more papers by this authorAhmet Güler
Department of Animal Science, Faculty of Agriculture, Ondokuz Mayıs University, Samsun, Turkey
Search for more papers by this authorCorresponding Author
Şengül Alpay Karaoğlu
Department of Biology, Faculty of Arts and Sciences, Recep Tayyip Erdoğan University, Rize, Turkey
Search for more papers by this authorNurettin Yayli
Faculty of Pharmacy, Karadeniz Technical University, Trabzon, Turkey
Search for more papers by this authorİshak Erik
Faculty of Pharmacy, Karadeniz Technical University, Trabzon, Turkey
Search for more papers by this authorBüşra Korkmaz
Faculty of Pharmacy, Karadeniz Technical University, Trabzon, Turkey
Search for more papers by this authorRahşan Akpinar
Laboratory of Bee Diseases, Veterinary Control Institute, Samsun, Turkey
Search for more papers by this authorArif Bozdeveci
Department of Biology, Faculty of Arts and Sciences, Recep Tayyip Erdoğan University, Rize, Turkey
Search for more papers by this authorŞeyma Suyabatmaz
Department of Biology, Faculty of Arts and Sciences, Recep Tayyip Erdoğan University, Rize, Turkey
Search for more papers by this authorNevzat Batan
Maçka Vocational School, Karadeniz Technical University, Trabzon, Turkey
Search for more papers by this authorAydın Yeşilyurt
Tonya Vocational School, Trabzon University, Trabzon, Turkey
Search for more papers by this authorSelma Kaya
Laboratory of Bee Diseases, Veterinary Control Institute, Samsun, Turkey
Search for more papers by this authorCevat Nisbet
Department of Biochemistry, Faculty of Veterinary Medicine, Ondokuz Mayıs University, Samsun, Turkey
Search for more papers by this authorAhmet Güler
Department of Animal Science, Faculty of Agriculture, Ondokuz Mayıs University, Samsun, Turkey
Search for more papers by this authorAbstract
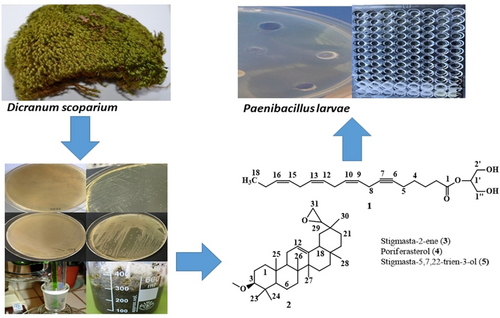
Bacterial diseases, such as American Foulbrood (AFB) and European Foulbrood (EFB), are known to have catastrophic effects on honey bees (if left to spread, can wipe out entire colonies), leading to severe financial losses in the beekeeping industry. The aim of this study was to evaluate the pharmacological properties of methanol extract and its fractions (ethyl acetate, hexane, water) derived from Dicranum scoparium Hedw., which could be utilized as a potential drug to prevent the bacterial diseases (AFB and EFB) affecting the honey bees. For this purpose, crude methanol extract and ethyl acetate/hexane/water fractions were prepared from the aerial part of D. scoparium, collected from Trabzon province. Bio-guided fractionation of the extract and its fractions led to the first-time isolation of five compounds. The structure of all compounds was elucidated by nuclear magnetic resonance (NMR) spectroscopy, ultraviolet (UV) spectral analysis, Fourier-transform infrared spectroscopy (FT-IR), liquid chromatography quadrupole time-of-flight mass spectroscopy (LC-QToF-MS), and by comparison of their NMR data with that of literature. The analysis of these compounds revealed significant antibacterial and sporicidal activities against bacteria causing larval diseases in honey bees. The antibacterial activity of these compounds ranged from 0.6 to 60 μg/mL against AFB and EFB causing bacteria. Therefore, the natural raw extract and fractions of D. scoparium could be used as potential therapeutic agents against bacterial agents affecting honey bees.
Graphical Abstract
Open Research
Data Availability Statement
Research data are not shared.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| cbdv202100887-sup-0001-misc_information.pdf481.4 KB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1R. A. Morse, K. Flottum, ‘Honey Bee Pests, Predators and Diseases’, Third Edition. AI, Root Company, Medina, Ohio, USA., 1997, 718 pp. ISBN: 0936028106.
- 2R. A. Morse, R. Nowogrodzki, ‘Honey Bee Pests, Predators and Diseases’, 2nd Ed. Cornstock Publishing Associates, USA, 1990, 474. ISBN: 0801424062.
- 3D. Yue, M. Nordhoff, L. H. Wieler, E. Genersch, ‘Fluorescence in situ hybridization (FISH) analysis of the interactions between honeybee larvae and Paenibacillus larvae, the causative agent of American foulbrood of honeybees (Apis mellifera)’, Environ. Microbiol. 2008, 10, 1612–1620.
- 4C. J. Brødsgaard, W. Ritter, H. Hansen, ‘Response of in vitro reared honey bee larvae to various doses of Paenibacillus larvae larvae spores’, Apidologie 1998, 29, 569–578.
- 5L. Bailey, B. V. Ball, ‘Honey bee pathology’, 2nd ed. Sidcup, United Kingdom: Harcourt Brace Jovanovich, 1991. p. 36–41. eBook ISBN: 9781483288093.
- 6C. Ashour, F. G. Priest, M. D. Collins, ‘Molecular identification of rRNA group 3 bacilli (Ashour, Farrow, Wallbanks and Collins) using a PCR probe test. Proposal for the creation of a new genus Paenibacillus’, Antonie van Leeuwenhoek 1992, 64, 253–260.
- 7S. P. Djordjevic, W. A. Forbes, L. A. Smith, M. A. Hornitzky, ‘Genetic and biochemical diversity among isolates of Paenibacillus alvei cultured from Australian honeybee (Apis mellifera) colonies’, Appl. Environ. Microbiol. 2000, 66, 1098–1106.
- 8R. Campos Herrera, F. E. El-Borai, R. J. Stuart, J. H. Graham, L. W. Duncan, ‘Entomopathogenic nematodes, phoretic Paenibacillus spp., and the use of real-time quantitative PCR to explore soil food webs in Florida citrus groves’, J. Invertebr. Pathol. 2011, 108, 30–39.
- 9A. M. Alippi, Bacterial diseases. In: Colin M. E. (ed.), Ball B. V. (ed.), Kilani M. (ed.).’Bee disease diagnosis. Zaragoza: CIHEAM, (1999). p. 31. (Options Méditerranéennes: Série B. Etudes et Recherches; n. 25). Course on Bee Disease Diagnosis 1997/05/19-30, Tunis (Tunisia).
- 10P. J. Elzen, D. Westervelt, D. Causey, J. Ellis, H. R. Hepburn, P. Neumann, ‘Method of application of tylosin, an antibiotic for American foulbrood control, with effects on small hive beetle (Coleoptera: Nitidulidae) populations’, J. Econ. Entomol. 2002, 95, 1119–1122.
- 11J. Kochansky, D. A. Knox, M. Feldlaufer, J. S. Pettis, ‘Screening alternative antibiotics against oxytetracycline-susceptible and -resistant Paenibacillus larvae’, Apidologie 2001, 32, 215–222.
- 12E. Genersch, ‘American Foulbrood in honeybees and its causative agent, Paenibacillus larvae’, J. Invertebr. Pathol. 2010, 103, 110–19.
- 13K. Raymann, Z. Shaffer, N. A. Moran, ‘Antibiotic exposure perturbs the gut microbiota and elevates mortality in honeybees’, PLoS Biol. 2017, 15, e2001861.
- 14Ż. Bargańska, M. Ślebioda, J. Namieśnik, ‘Determination of antibiotic residues in honey’, TrAC Trends Anal. Chem. 2011, 30, 1035–1041.
- 15T. Miyagi, C. Y. Peng, R. Y. Chuang, E. C. Mussen, M. S. Spivak, R. H. Doi, ‘Verification of oxytetracycline-resistant American foulbrood pathogen Paenibacillus larvae in the United States’, J. Invertebr. Pathol. 2000, 75, 95–96.
- 16R. Waite, S. Jackson, H. Thompson, ‘Preliminary investigations into possible resistance to oxytetracycline in Melissococcus plutonius, a pathogen of honeybee larvae’, Lett. Appl. Microbiol. 2003, 36, 20–24.
- 17M. Spivak, G. S. Reuter, ‘Resistance to American foulbrood disease by honey bee colonies Apis mellifera bred for hygienic behavior’, Apidologie 2001, 32, 555–565.
- 18M. Hornitzky, ‘Fatty acids - an alternative control strategy for honeybee diseases’, Rural Industries Research and Development Corporation Publication, 2003. DAN-193A.
- 19H. Beims, J. Wittmann, B. Bunk, C. Spröer, C. Rohde, G. Günther, M. Rohde, W. von der Ohe, M. Steinert, ‘Paenibacillus larvae-Directed Bacteriophage HB10c2 and Its Application in American Foulbrood-Affected Honey Bee Larvae’, Appl. Environ. Microbiol. 2015, 81, 5411–5419.
- 20I. Alvarado, J. W. Margotta, M. M. Aoki, F. Flores, F. Agudelo, G. Michel, M. M. Elekonich, E. Abel-Santos, ‘Inhibitory effect of indole analogs against Paenibacillus larvae, the causal agent of American foulbrood disease’, J. Insect Sci. 2017, 17, 1–8.
- 21B. A. Daisley, A. P. Pitek, J. A. Chmiel, K. F. Al, A. M. Chernyshova, K. M. Faragalla, J. P. Burton, G. J. Thompson, G. Reid, ‘Novel probiotic approach to counter Paenibacillus larvae infection in honey bees’, The ISME J. 2020, 14, 476–491.
- 22L. Hedenäs, I. Bisang, ‘Key to European Dicranum species’, Herzogia 2004, 17, 179–197.
- 23R. M. Ros, V. Mazimpaka, U. Abou-Salama, M. Aleffi, T. L. Blockeel, M. Brugués, R. M. Cros, M. G. Dia, G. M. Dirkse, I. Draper, W. El-Saadawi, A. Erdağ, A. Ganeva, R. Gabriel, J. M. González-Mancebo, C. Granger, I. Herrnstadt, V. Hugonnot, K. Khalil, H. Kürschner, A. Losada-Lima, L. Luís, S. Mifsud, M. Privitera, M. Puglisi, M. Sabovljević, C. Sérgio, H. M. Shabbara, M. Sim-Sim, A. Sotiaux, R. Tacchi, A. Vanderpoorten, O. Werner, ‘Mosses of the Mediterranean, an annotated checklist’, Cryptogamie, Bryologie 2013, 34, 99–285.
- 24A. J. E. Smith, ‘The moss flora of Britain and Ireland’, Second Edition, 1012 pp., Cambridge University Press, Cambridge (2004) ISBN-10, 0-51122968-2.
- 25C. Borel, D. H. Welti, I. Fernandez, M. Colmenares, ‘Dicranin, an antimicrobial and 15-lipoxygenase inhibitor from the moss Dicranum scoparium’, J. Nat. Prod. 1993, 56, 1071–1077.
- 26B. G. Österdahl, T. Laitalainen, M. Mosihuzzaman, D. Johnels, S. Hellberg, M. Sjöström, S. Wold, R. Mahedevan, ‘Chemical Studies on Bryophytes. 23. 13C-NMR Analysis of a Biflavone from Dicranum scoparium’, Act. Chem. Scand B 1983, 37, 69–71.
10.3891/acta.chem.scand.37b-0069 Google Scholar
- 27H. Geiger, A. Voigt, H. Zinsmeister, J. López-Sáez, M. Pérez-Alonso, A. Velasco-Negueruela, ‘The biflavones of Dicranum scoparium (Dicranaceae)’, Z. Naturforsch. 1993, 48, 952–955.
- 28E. M. Altuner, B. Çetin, ‘Antimicrobial Activity of Thuidium delicatulum (Bryopsida) Extracts’, Kafkas Üniv. Fen Bil. Enst. Derg. 2009, 2, 85–92.
- 29V. Nikolajeva, L. Liepina, Z. Petrina, G. Krumina, M. Grube, I. Muiznieks, ‘Antibacterial activity of extracts from some Bryophytes’, Adv Microbiol. 2012, 2, 345–353.
10.4236/aim.2012.23042 Google Scholar
- 30G. Bhadauriya, S. Singh, K. S. Rathore, ‘Phytochemical screening and total phenolic content in the extract of bryophyte Plagiochasma appendiculatum and Dicranum scoparium’, E. C. J. 2018, 19, 175–181.
- 31F. Savaroglu, C. Filik İşçen, A. P. Öztopcu Vatan, S. Kabadere, S. İlhan, R. Uyar, ‘Screening of antimicrobial, cytotoxic effects and phenolic compounds of the moss Dicranum scoparium’, BioDiCon. 2018, 11, 87–94.
- 32G. Abay, M. Altun, Ö. C. Karakoç, F. Gül, I. Demirtas, ‘Insecticidal activity of fatty acid-rich Turkish bryophyte extracts against Sitophilus granarius (Coleoptera: Curculionidae)’, Comb. Chem. High Throughput Screening 2013, 16, 806–816.
- 33B. G. Osterdahl, ‘Chemical studies on bryophytes. 20. A new branched flavonoid-O-triglycoside from Dicranum scoparium’, Act Chem Scand B 1978, 32, 714–716.
- 34A. Basile, S. Giordano, J. A. Lopez-Saez, R. C. Cobianchi, ‘Antibacterial activity of pure flavonoids isolated from mosses’, Phytochemistry 1999, 52, 1479–1482.
- 35A. Basile, S. Sorbo, J. A. López-Sáez, R. Castaldo Cobianchi, ‘Effects of seven pure flavonoids from mosses on germination and growth of Tortula muralis HEDW (Bryophyta) and Raphanus sativus L (Magnoliophyta)’, Phytochemistry 2003, 62, 1145–1151.
- 36T. Ichikawa, M. Namikawa, K. Yamada, K. Sakai, K. Kondo, ‘Novel cyclopentenonyl fatty acids from mosses Dicranum scoparium and Dicranum japonicum’, Tetrahedron Lett. 1983, 24, 3337–3340.
- 37No name of inventor, ‘Antimicrobial higher unsaturated fatty acid derivatives’, Jpn. Kokai Tokkyo Koho. 1984, JP 59154993A 19840904.
- 38T. Ichikawa, K. Yamada, M. Namikawa, K. Sakai, K. Kondo, ‘New cyclopentenonyl fatty acids from Japanese mosses’, J. Hattori Bot. Lab. 1984, 56, 209–213.
- 39G. Lindberg, B. G. Osterdahl, E. Nilsson, ‘Chemical studies on bryophytes. 16. 5′,8′′-Biluteolin, a new biflavone from Dicranum scoparium’, Chem. Scr. 1974, 5, 140–144.
- 40G. Abay, M. Altun, S. Koldas, A. R. Tufekci, I. Demirtas, ‘Determination of Antiproliferative Activities of Volatile Contents and HPLC Profiles of Dicranum scoparium (Dicranaceae, Bryophyta)’, Comb. Chem. High Throughput Screening 2015, 18, 453–463.
- 41D. Saxena, K. Harinder, ‘Uses of bryophytes’, Resonance 2004, 9, 56–65.
10.1007/BF02839221 Google Scholar
- 42J. Shaw, K. Renzaglia, ‘Phylogeny and diversification of bryophytes’, Am. J. Bot. 2004, 91, 1557–1581.
- 43T. M. Karpıńskı, A. Adamczak, ‘Antibacterial activity of ethanolic extracts of some moss species’, Herba Pol. 2017, 63, 11–17.
10.1515/hepo-2017-0014 Google Scholar
- 44G. R. Narayan, V. Kartik, P. Manoj, P. S. Singh, G. Alka, ‘Antibacterial activities of ethanolic extracts of plants used in folk medicine’, IJRAP. 2010, 1, 529–535.
- 45V. P. Flanagan, A. Ferretti, J. M. Ruth, ‘Characterization of two steroidal olefins in nonfat dry milk’, Lipids 1974, 9, 471–475.
- 46J. P. Valerie, W. Fenical, ‘Toxic acetylene-containing lipids from the red marine alga Liagora farinosa lamouroux’, Tetrahedron Lett. 1980, 21, 3327–3330.
- 47V. Chaimanee, U. Thongtue, N. Sornmai, S. Songsri, J. S. Pettis, U. Thongtue, N. Sornmai, S. Songsri, J. S. Pettis, ‘Antimicrobial activity of plant extracts against the honeybee pathogens, Paenibacillus larvae and Ascosphaera apis and their topical toxicity to Apis mellifera adults’, J. Appl. Microbiol. 2017, 123, 1160–1167.
- 48E. M. Altuner, K. Canli, I. Akata, ‘Antimicrobial Screening of Calliergonella cuspidata, Dicranum polysetum and Hypnum cupressiforme’, J. Pure Appl. Microbiol. 2014, 8, 539–545.
- 49C. Srinivasulu, M. Ramgopal, G. Ramanjaneyulu, C. M. Anuradha, C. S. Kumar, ‘Syringic acid (SA)-A Review of Its Occurrence, Biosynthesis, Pharmacological and Industrial Importance’, Biomed. Pharmacother. 2018, 108, 547–157.
- 50M. Matin Yekta, S. H. Reza Alavi, ‘New Triterpenoids from Peucedanum ruthenicum’, Iranian J. Pharmaceutical Sci. 2008, 4, 289–294.
- 51A. Vierengel, G. Kohn, O. Vandekerkhove, E. Hartmann, ‘9-Octadecen-6-ynoic acid from Riccia fluitans’, Phytochem. 1987, 26, 2101–2102.
- 52M. Rempt, B. Schneider, G. A. Pohnert, ‘Reactive Conjugated Allene Involved in the Biosynthesis of Volatile Oxylipins in the Moss Dicranum scoparium’, Org. Lett. 2011, 13, 3229–3231.
- 53J. W. Blunt, M. H. G. Munro, ‘Carbon-13 NMR spectra of some tetra- and pentacyclic triterpene methyl ethers’, Org. Magn. Reson. 1980, 13, 26–27.
- 54R. L. Baxter, K. C. Price, G. Fenwick, ‘Sapogenin structure: analysis of the carbon-13 and proton NMR spectra of soyasapogenol B’, J. Nat. Prod. 1990, 53, 298–302.
- 55H. L. Chiu, J. H. Wu, Y. T. Tung, T. H. Lee, S. C. Chien, Y. H. Kuo, 'Triterpenoids and Aromatics from Derris laxiflora’, J. Nat. Prod. 2008, 71, 1829–1832.
- 56J. Hu, X. Shi, J. Chen, H. Huang, C. Zhao, ‘Cytotoxic taraxerane triterpenoids from Saussurea graminea’, Fitoterapia 2012, 83, 55–59.
- 57N. N. Okoye, D. L. Ajaghaku, H. N. Okeke, E. E. Ilodigwe, C. S. Nworu, F. B. C. Okoye, ‘Beta-Amyrin and alpha-amyrin acetate isolated from the stem bark of Alstonia boonei display profound anti-inflammatory activity’ Pharm. Biol. 2014, 52, 1478–1486.
- 58J. Otaka, M. Komatsu, Y. Miyazaki, Y. Futamura, H. Osada, ‘Two new triterpenoids from the roots of Pinus densiflora’ Biosci. Biotechnol. Biochem. 2017, 81, n 449–452.
- 59R. M. Sabrin Ibrahim, S. Ehab Elkhayat, G. A. Mohamed, A. I. M. Khedr, M. A. Fouad, M. H. R. Kotb, A. R. Samir, ‘Aspernolides F and G, new butyrolactones from the endophytic fungus Aspergillus terreus’, Phytochem. Lett. 2015, 14, 84–90.
- 60A. M. Alippi, F. J. Reynald, ‘Inhibition of the growth of Paenibacillus larvae, the causal agent of American foulbrood of honeybees, by selected strains of aerobic spore-forming bacteria isolated from apiarian sources’, J. Invertebr. Pathol. 2006, 91, 141–146.
- 61D. Mennie, C. F. Moffat, A. S. McGill, ‘Identification of sterene compounds produced during the processing of edible oils’, J. High Resol. Chromatography 1994, 17, 831–838.
- 62O. B. Acikara, G. S. Citoğlu, S. Dall′Acqua, H. Ozbek, J. Cvačka, M. Žemlička, K. Šmejkal, ‘Bioassay-guided isolation of the antinociceptive compounds motiol and β-sitosterol from Scorzonera latifolia root extract’, Pharmazie 2014, 69, 711–714.
- 63D. C. De Graaf, A. M. Alippi, K. Antúnez, K. A. Aronstein, G. E. Budge, D. De Koker, L. De Smet, D. W. Dingman, J. D. Evans, L. J. Foster, A. Funfhaus, E. Garcia-Gonzalez, A. Gregorc, H. Human, K. D. Murray, B. K. Nguyen, L. Poppinga, M. Spivak, D. VanEngelsdorp, S. Wilkins, E. Genersch, ‘Standard methods for American foulbrood research’, J. Api. Res. 2013, 52, 1–27.
- 64E. Forsgren, G. E. Budge, J. D. Charrière, M. A. Z. Hornitzky, ‘Standard methods for European foulbrood research. In Dietemann V, Ellis JD, Neumann P (Eds). The Coloss Beebook: Volume II: Standard methods for Apis mellifera pest and pathogen research’, J. Api. Res. 2013, 52, 1–14.
- 65E. Genersch, A. Ashiralieva, I. Fries, ‘Strain- and genotype-specific differences in virulence of Paenibacillus larvae subsp. larvae, a bacterial pathogen causing American foulbrood disease in honeybees’, Appl. Environ. Microbiol. 2005, 71, 7551–7555.
- 66OIE, ‘American foulbrood of honey bees (infection of honey bees with Paenibacillus larvae’, OIE Terrestrial Manual, 2018, p. 719–735.
- 67E. Sevim, Y. Baş, G. Çelik, M. Pınarbaş, A. Bozdeveci, T. Özdemir, R. Akpınar, N. Yaylı, Ş. A. Karaoğlu, ‘Antibacterial activity of bryophyte species against Paenibacillus larvae isolates’, Turkish J. Vet. Anim. Scien. 2017, 41, 521–531.
- 68EUCAST, ‘The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters’, Vers. 5.0. valid from 2015–01-01. 2014. http://www.eucast.org.
- 69A. Okayama, T. Sákogawa, C. Nakajima, T. Hayama, ‘Sporicidal activities of disinfectants on Paenibacillus larvae’, J. Vet. Med. Sci. 1997, 59, 953–954.
- 70A. Meda, C. E. Lamien, M. Romito, J. Millogo, O. G. Nacoulma, ‘Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity’, Food Chem. 2005, 91, 571–577.
- 71V. L. Singleton, R. Orthofer, R. M. Lamuela-Raventós, ‘Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent’, Methods Enzymol. 1999, 299, 152–178.
- 72C. C. Chang, M. H. Yang, H. M. Wen, J. C. Chern, ‘ Estimation of total flavonoid content in propolis by two complementary colorimetric methods’, J. Food Drug Anal. 2002, 10, 178–182.
- 73T. Yooboon, K. Kuramitsu, V. Bullangpoti, Y. Kainoh, S. Furukawa, ‘Cytotoxic effects of β-asarone on Sf9 insect cells’, Arch. Insect Biochem. Physiol. 2019, 102, e21596.
- 74R. F. Teixeira Corrêa, D. M. P. Ardisson-Araújo, R. G. Monnerat, B. M. Ribeiro, ‘Cytotoxicity Analysis of Three Bacillus thuringiensis Subsp. israelensis δ-Endotoxins towards Insect and Mammalian Cells’, PLoS One 2012, 7, e46121.
- 75Y. Asakawa, ‘Recent advances in Phytochemistry of bryophytes-acetogenins, terpenoids and bis(bibenzyl)s from selected Japanese, Taiwanese, New Zealand, Argentinean and European liverworts’, Phytochemistry 2001, 56, 297–312.