Role of Exosome in Solid Cancer Progression and Its Potential Therapeutics in Cancer Treatment
[Correction added on June 26, 2025 after first online publication. The co-author name has been corrected from ‘Assela’ to ‘Aseela’ in this version.]
ABSTRACT
Background
Exosomes are extracellular vesicles ranging from 40 to 100 nm in diameter that mediate intercellular communication by transferring proteins, lipids, nucleic acids, and other metabolites. In the context of cancer, exosomes influence the tumor microenvironment by carrying regulatory RNAs such as miRNA, circRNA, and lncRNA. They originate from various cells, including adipocytes, fibroblasts, and hepatocellular carcinoma (HCC) cells, and can either promote or inhibit cancer progression through pathways like MAPK and PI3K-Akt.
Aim
This review aims to explore the role of exosomes in the progression of solid cancers, emphasizing their self-induced activation mechanisms and how they modulate tumor behavior.
Methodology
A comprehensive review of recent literature was conducted, focusing on studies that investigated the biological functions of exosomes in solid tumor progression, including their molecular cargo, cellular origin, and involvement in signaling pathways.
Results
Findings from multiple studies indicate that cancer-derived exosomes contribute to tumor proliferation, metastasis, and therapy resistance by enhancing communication within the tumor microenvironment. These vesicles activate oncogenic pathways and can serve as biomarkers or therapeutic targets due to their role in disease modulation.
Conclusion
Exosomes play a pivotal role in solid cancer progression and offer significant potential in advancing our understanding of tumor biology. Their capacity to influence key signaling pathways and facilitate intercellular communication makes them promising candidates for novel diagnostic and therapeutic strategies.
1 Introduction
Cancer, once relatively uncommon, has seen a significant rise in incidence over the past two decades, driven by lifestyle changes, evolving habits, and increased life expectancy [1]. It is a disease characterized by gene mutations that disrupt normal cellular processes of replication, differentiation, and death, leading to the initiation and progression of tumors [2]. As tumors grow, they recruit normal cells from the surrounding environment, causing structural and cellular changes that impact tumor biology and response to treatment [3]. Standard treatments, such as surgery, radiation, and chemotherapy, can damage healthy cells and cause toxic side effects [4]. Thus, developing more effective and precise cancer therapies is crucial for enhancing patient outcomes.
The tumor microenvironment (TME) is a complex network comprising malignant cells and various supportive components, including fibroblasts, adipocytes, endothelial cells, immune cells, and the extracellular matrix (ECM) [5]. Cells within the TME interact through direct contact and the release of signaling molecules like cytokines, chemokines, and extracellular vesicles (EVs) such as exosomes [6]. Exosomes, once released, are internalized by recipient cells through endocytosis or receptor-mediated interactions, facilitating intercellular communication [7, 8]. This signaling can drive immune evasion, alter stromal cell behavior, and remodel the ECM, promoting tumor progression [9-11].
Exosomes are EVs released via exocytosis, resulting in a lipid bilayer orientation similar to the plasma membrane. Markers of exosome biogenesis include proteins such as Rab GTPases and ESCRT proteins, with commonly recognized surface markers being CD9, CD81, CD63, flotillin, TSG101, ceramide, and Alix [12]. Exosomes, classified based on size and origin, range from 40 to 100 nm in diameter and originate from endosomes. Larger EVs like macrovesicles and apoptotic bodies are derived from the plasma membrane [13]. Exosomes can also be categorized based on their cellular origin, such as adipocyte-derived, fibroblast-derived, or stem cell-derived exosomes, each playing roles as either cancer promoters or inhibitors [14-17].
Exosomes have emerged as a significant focus of research in cancer biology due to their dual nature. They are implicated in tumor progression through the transfer of miRNA, circRNA, and lncRNA, which activate pathways like MAPK, RAS, PI3K-Akt, and Wnt/β-catenin, promoting cancer proliferation and metastasis [15, 18]. Exosomes also contribute to tumor immunosuppression by altering immune responses, such as transforming T-cells into Treg cells and inducing NK cell exhaustion [19-21]. Conversely, some exosomes inhibit cancer growth, as seen with umbilical cord mesenchymal stem cell (MSC)-derived exosomes that suppress hepatocellular carcinoma (HCC) proliferation, induce apoptosis, and reduce angiogenesis [14]. Other exosomes, such as those containing circ_0051443, promote apoptosis and cell cycle arrest in cancer cells, highlighting their potential therapeutic role [22].
This review aims to update our present understanding of the biogenesis and functions of tumor-derived exosomes, emphasizing their critical contribution to TME signaling and their influence on solid cancer progression, focusing on brain, liver, and breast cancers. These cancers were selected for their obvious clinical and biologic relevance: Brain tumors, i.e., glioblastoma (GBM), represent a particular therapeutic challenge due to the blood–brain barrier (BBB) and immunosuppressive TME, but also offer the opportunity for exosome-mediated drug delivery, as discussed under therapeutic approaches [23, 24]. Liver cancers, including HCC, are intimately associated with chronic inflammation and fibrosis, where exosomes facilitate immune evasion and stromal remodeling to sustain a pro-tumorigenic niche [25, 26]. Breast cancer, with its astounding heterogeneity, is an exemplary model of exosome-mediated communication between tumor cells and stromal components such as adipocytes, macrophages, and fibroblasts, which fuel metastasis and therapy resistance [27, 28]. Collectively, these malignancies highlight diverse microenvironments and exosome-mediated processes that are primed for biomarker development and therapeutic innovation. By examining these environments, we interpret the double-edged nature of exosomes as both architects of cancer and therapeutics, and their potential to refine targeted therapy strategies across solid tumors.
1.1 Biogenesis and Signaling Pathways of Tumor-Derived Exosomes
Tumor-derived exosomes (TDEs) have gained significant attention in cancer research due to their immunoregulatory roles and involvement in tumor pathophysiology. The biogenesis of exosomes involves four main stages: cargo sorting, endocytosis, multivesicular body (MVB) formation, and exosome release. This process begins with the recruitment of various molecules—proteins, nucleic acids, and lipids—into early endosomes. Cargo sorting is regulated by distinct mechanisms; for example, proteins often require monoubiquitination, while miRNAs are guided into exosomes by binding to heterogeneous nuclear ribonucleoprotein A2B1 (hnRNPA2B1) [29-32].
Early endosomes form through plasma membrane invagination and undergo maturation into late endosomes, which involves changes in membrane composition, such as the conversion of sphingomyelin to ceramides and the replacement of Rab5 with Rab11, facilitating endosomal trafficking [33-36]. During this maturation, inward budding of the endosomal membrane creates dynamic MVBs. These MVBs can either fuse with lysosomes, leading to cargo degradation, or with the plasma membrane, releasing their intraluminal vesicles (ILVs) as exosomes into the extracellular space [37-40] as shown in Figure 1.
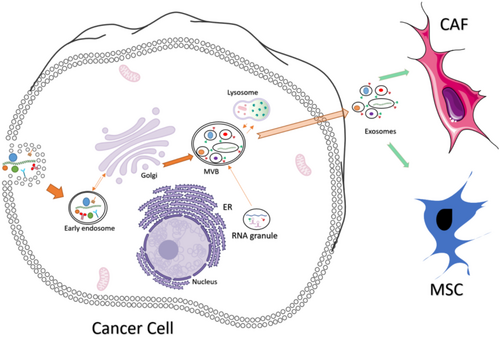
Exosome synthesis and release involve complex pathways, including Endosomal Sorting Complexes Required for Transport (ESCRT)-dependent and ESCRT-independent mechanisms. Released exosomes deliver their cargo—such as DNA, microRNA, and proteins—to recipient cells through mechanisms like direct fusion with the plasma membrane, surface protein binding, or endocytosis [41]. By utilizing these mechanisms, TDEs significantly contribute to intercellular communication, supporting tumor growth, proliferation, angiogenesis, and metastasis. They deliver immunosuppressive molecules to stromal and immune cells, dampening immune responses against cancer [42].
Exosomes derived from tumors express immunosuppressive proteins such as programmed death-ligand 1 (PD-L1), which inhibits T-cell activation [43, 44]. TDEs also mediate immune suppression through the FAS/FASL pathway, promoting T-cell apoptosis and signaling via exosomal TGF-β1 [45]. The ESCRT-associated protein ALIX regulates PD-1 expression on cell membranes; its downregulation increases PD-1 expression in breast cancer cells, enhancing immune evasion [46]. Cancer-associated fibroblast (CAF)-derived exosomes overexpress miR-92, which upregulates PD-L1 in breast cancer cells, linking reduced cell division and increased T-cell apoptosis [47]. TDE signaling further suppresses natural killer (NK) cells by inhibiting interleukin-2 and transporting heat shock protein-72 to regulate myeloid-derived suppressor cells (MDSCs) via the Signal transducer and activator of transcription 3 (STAT3) pathway. Additionally, TDEs transfer epidermal growth factor receptor (EGFR) to adjacent macrophages, contributing to immune suppression [46]. Exosomes from MSCs influence tumor-associated macrophages (TAMs), converting them into immunosuppressive type-2 macrophages that enhance arginase-1 activity and interleukin-10 secretion, promoting malignancy and immune suppression at the tumor site [48]. Exosomes deriving from MSCs have a large amount of semaphorins, TGF-β and C1Q which incentivize the expression of PD-L1 in macrophages [49]. The epithelial–mesenchymal transition (EMT) also stimulates exosome biogenesis and release in tumor cells. CAFs secrete TGF-β1 through the TGF-β1-SMAD pathway, facilitating EMT and supporting exosome-mediated signaling [50-52]. Exosomes from these fibroblasts can activate the Wnt pathway in breast cancer cells, promoting metastasis [53].
Exosome-mediated delivery of miRNA-rich cargo activates key pathways such as Ras, PI3K-Akt, and MAPK, driving cancer proliferation [15]. For example, exosomes containing miR-374a-5p, miR-200b-3p, and miR-21-5p activate the Wnt/β-catenin and PI3K/Akt pathways in HCC, enhancing tumor aggressiveness [15]. miR-17-5p, another exosomal miRNA, promotes proliferation by inhibiting MAPK9 and suppressing the G1/S cell cycle checkpoint [54]. Downregulation of critical mRNAs, like GADD45A, further destabilizes genomic integrity, contributing to aggressive cancer phenotypes [15]. Furthermore, genes associated with cell proliferation and collagen production were found to be upregulated, whereas those related to immune functions, such as CLEC1B and CLEC4G, were downregulated [16].
1.2 Tumor Microenvironment
The TME is a dynamic and intricate ecosystem that evolves continuously to support cancer development. It consists of diverse cellular and non-cellular components that play pivotal roles in all stages of carcinogenesis. The TME typically comprises immune cells, stromal cells, blood vessels, and the ECM, though its composition varies by tumor type [55, 56]. The interaction between cancer cells and the surrounding environment fosters tumor survival, local invasion, and metastatic spread from the early stages of tumor growth, illustrated in Figure 2 [55].
1.2.1 Immune Cells in the TME
Immune cells within the TME can either fight against the tumor or facilitate its growth, contributing to a highly dynamic immune landscape. Tumors can be classified into three types based on immune infiltration: immune-infiltrated, immune-excluded (where immune cells are at the tumor's periphery but have not penetrated it), and immune-silent (with a complete lack of immune cell infiltration) [55, 57]. In immune-infiltrated tumors, cytotoxic T-cells move via a multistep process from the bloodstream to the tumor, signifying an active immune response [58]. However, tumor-associated immune cells, such as TAMs, often support tumor progression by promoting angiogenesis, suppressing T-cell function, and aiding metastasis [55, 59].
1.2.2 Stromal Cells and the ECM
Stromal cells, such as CAFs, adipocytes, and endothelial cells, play critical roles in tumor growth, angiogenesis, and metastasis. CAFs are particularly influential in restricting immune cell access to the tumor and secreting factors that support cancer cell survival [60]. Adipocytes in adipocyte-rich environments, such as breast cancer, release metabolic substrates, and cytokines that promote cancer cell proliferation and migration [61, 62].
In liver cancer, hepatic stellate cells (HSCs) transform into myofibroblasts in response to tissue injury, contributing to the fibrotic and inflammatory environment that supports HCC development [63, 64]. Endothelial cells (ECs) in the vascular endothelium are essential for tumor angiogenesis, which restores oxygen and nutrient supply to growing tumors that have outgrown passive diffusion [65]. This angiogenic process is often driven by hypoxia and is critical to the survival of tumors larger than 1–2 mm3 [55, 65].
The ECM, a three-dimensional network of macromolecules such as collagen, fibronectin, elastin, laminin, and proteoglycans, provides structural support to the tumor and plays a crucial role in facilitating metastasis [66]. ECM consists of collagen, fibronectin, elastin, laminin, glycoproteins, and proteoglycans [67]. In solid tumors, the ECM forms extensive deposits, making up as much as 60% of the tumor's mass [55, 68].
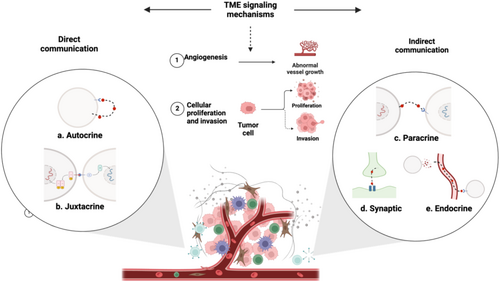
In the TME, cells interact through various signaling mechanisms that promote carcinogenesis. These interactions can be direct, such as autocrine signaling where a cell binds to its own secreted messenger (Figure 3a) [69], or juxtacrine signaling where membrane-bound ligands on one cell interact with receptors on an adjacent cell (Figure 3b) [70]. Alternatively, indirect communication occurs locally through paracrine –such as VEGF-driven angiogenesis (Figure 3c) [71, 72], or synaptic signaling, particularly in neural cancers such as brain cancer [73] (Figure 3d). Additionally, cells communicate over longer distances through endocrine signaling mechanisms, including the transfer of biological information via exosomes [74-76], or by releasing signaling molecules and mediators such as cytokines, chemokines, and growth factors (Figure 3e) [75]. Consequently, these mechanisms modulate immune responses, reprogram stromal cells, and remodel the ECM, driving tumor proliferation, invasion, metastasis, and angiogenesis [9, 72, 77]. As tumors grow, hypoxia and metabolic waste accumulation create a stressful microenvironment that triggers angiogenesis to restore oxygen and nutrient levels [65]. Hypoxia-induced factors, such as hypoxia-inducible factor 1-alpha (HIF-1α), stimulate the secretion of pro-angiogenic factors like VEGF, promoting the formation of new blood vessels. This newly formed vasculature is often abnormal, contributing to inefficient blood flow and exacerbating the hypoxic and acidic conditions within the TME, further driving tumor progression [65].
1.2.3 Extracellular Vesicles in TME
EVs are crucial in promoting the process of cell–cell communication within the TME, initiating a cross talk between cancer cells and stromal cells [78]. EVs are classified into two major subgroups-small vesicles budding directly from the endosomal compartments primarily called as exosomes and larger vesicles blebbing off the plasma membrane primarily termed as microvesicles [78]. EVs pathogenic process involves the transfer of the contained biomolecules either through fusing with the plasma membrane, endocytosis or ligand-receptor interactions [79], promoting tumor growth, invasion, metastasis, angiogenesis, immunological modifications, EMT and inflammation, via transforming tumor cells, tumor-associated cells (endothelial, fibroblast, macrophages, and leucocytes) or normal cells within the TME or at cells in distant locations developing pre-metastatic niches [76, 80, 81]. The biomolecules utilized by the EVs could include proteins, lipids, DNAs, and non-coding RNA [79]. For instance, EVs derived from breast cancer cells had an upregulated display of VEGF which stimulated the VEGFR-signaling within the endothelial cells, with enhanced total VEGF secretion under hypoxic conditions promoting angiogenesis [81], or breast cancer cells derived EVs promoted epithelial to mesenchymal transition (EMT) by stimulating TGF-β signaling due to the transfer of TGF-beta II receptor, which is expressed on the EVs, promoting cancer stemness, metastasis and CD8+ T cells exhaustion by breast cancer cells [82]. However, most of the cell–cell communications are dominated by the non-coding RNA (ncRNA) (miRNA, lnRNA, and circRNA) [83, 84]. For instance, EVs released by CAFs surrounding colorectal adenocarcinoma cells (COAD) promoted angiogenesis by delivering miR-135-5p, which negatively regulated FOXO1 expression, further inducing COAD cells to promote proliferation, migration and angiogenesis of endothelial cells causing angiogenesis [85]. The formation of the metastatic niche at a secondary location is primarily influenced by the EVs. For instance, the study conducted by Gener Lahav et al. investigated the potential of melanoma-derived EVs to form secondary metastatic niches. They identified melanoma-derived EVs increased proinflammatory signaling within astrocytes and lung fibroblast cells along with activated CAF characteristics, establishing hospitable metastatic niches [86]. Additionally, EVs derived from cells other than cancer cells promote tumorigenesis and modulate the TME [25]. The study conducted by Zhou et al. identified human umbilical mesenchymal stem cells (hU-MSC) promoted proliferation, migration and EMT within breast cancer cells through the ERK pathway [25]. Furthermore, EVs are capable of destructing the blood-brain barrier (BBB) promoting brain metastasis [87]. For instance, Tominaga et al. identified miR-181c from the breast cancer cells promoted the destruction of BBB through the delocalization of actin through the downregulation of 3-phosphoinositide-dependent protein kinase-1 (PDPK1), which resulted in the downregulation of phosphorylation of cofilin, leading to the modulation of actin dynamics, inducing brain metastasis. Therefore, EVs are derived from varied sources, such as cancer cells or from the surrounding cells, promoting cancer metastasis via modulating TME through cell-to-cell communication [87].
2 How EVs Control the Communication
Exosomes play a crucial role in cell–cell communication, traditionally mediated by mechanisms such as gap junctions, receptor/ligand interactions, and electrical or chemical signals. They help regulate cellular responses to the external environment. Numerous studies have confirmed the importance of exosomes in both physiological and pathological processes. TDEs are secreted in greater quantities by cancer cells than by normal cells, transferring tumor-associated microRNAs and other signaling molecules to target cells through exosome fusion with cell membranes [88]. This exchange facilitates tumor growth, metastasis, and various other tumor-promoting mechanisms, as illustrated in Figure 3 [89, 90].
For instance, in GBM multiforme, a highly aggressive brain tumor, exosomes have been shown to mediate specific communication between tumor cells and the surrounding non-tumor cells, such as glial cells, neurons, and vascular cells. Longitudinal time-lapse imaging revealed that glioma cells exchange EVs with different brain cell types to promote glioma growth. This communication is selective, with astrocytes or microglia/macrophages preferentially taking up debris from glioma cells. In a study where glioma-derived EVs were injected into Ai14 mice, no significant EV uptake was observed in the brains of these animals, suggesting cell-type specificity in EV-mediated communication [91].
The proteins carried by TDEs significantly influence the TME. Among these proteins are cell adhesion molecules such as integrins, annexins, tetraspanins, and proteases. For example, Annexin A2 (ANXA2) plays a key role in invasion, metastasis, angiogenesis, and cell proliferation, while CD44, a receptor for hyaluronic acid, osteopontin, and matrix metalloproteinases, contributes to tumor growth, cell motility, and angiogenesis. CD44 is also regarded as a marker for cancer stem cells, further linking exosome-mediated communication to malignancy [92].
TDEs play a crucial role not only in promoting tumor growth and progression but also in facilitating metastasis. For example, breast cancer-derived exosomes have been shown to transport inducers of the EMT, a critical step in metastasis [93]. During EMT, epithelial cells lose their polarity and cell–cell adhesion properties, acquiring mesenchymal traits that allow them to migrate through the bloodstream, invade distant tissues, and form metastatic colonies [94]. This transition also leads to ECM remodeling and the development of anti-apoptotic phenotypes and pre-metastatic niches [95]. Moreover, TDEs facilitate organ-specific metastasis. In gastric cancer, exosomes carrying EGFR have been implicated in promoting liver metastasis. These exosomes activate hepatocyte growth factor (HGF) by suppressing miR-26a/b expression, thereby creating a liver-like microenvironment. Elevated levels of HGF stimulate cancer cell proliferation through the activation of the c-MET receptor, further promoting.
3 Exosomes and the Hallmarks of Cancer
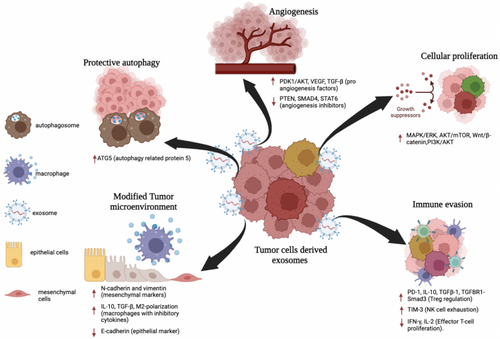
EVs including exosomes, microvesicles, and apoptotic bodies, have emerged as key players in cancer research due to their involvement in the communication between cancer cells and the TME. Exosomes, in particular, are stable particles that can travel throughout the body, facilitating tumor growth and spread. Research has shown that both TDEs and stromal cell-derived exosomes contribute to creating a supportive environment for cancer progression by modifying surrounding tissue [96]. EVs are essential for understanding the molecular mechanisms of cell communication within the TME, as they establish and maintain several cancer hallmarks, including promoting cell proliferation, resisting cell death, evading immune responses, reprogramming energy metabolism, and facilitating angiogenesis [97-99].
3.1 Promoting Cell Proliferation and Resisting Cell Death
EVs play a central role in cancer progression by inducing the transformation of normal cells into cancerous ones and supporting the phenotypic transformation of tumor cells. This transformation promotes tumor growth by stimulating proliferative signaling through pathways such as MAP/ERK, PI3K/AKT, and WNT. Sustained autocrine or paracrine signaling mediated by cancer EVs provides tumor cells with a proliferative advantage [100]. For example, EVs from GBM cells have been shown to enhance tumor growth by transferring oncogenic proteins to neighboring glioma cells, activating growth-promoting pathways. Studies involving GBM cells injected into mice demonstrated that EVs containing specific proteins enhanced tumor growth compared to EVs lacking those proteins [98]. Additionally, EVs from aggressive cancer cells, such as MDA-MB231 breast cancer cells, have been shown to transfer malignancy traits like anchorage-independent growth and survival under nutrient-limiting conditions to normal cells. EVs also play a critical role in resisting apoptosis. GBM EVs, for example, have been found to carry splicing factors that induce the expression of oncogenic gene isoforms in recipient cells, promoting cell survival. Additionally, microRNAs (miRNAs) in EVs, such as miR-1246 in breast cancer EVs and miR-205 in cholangiocarcinoma EVs, have been implicated in promoting cell proliferation and suppressing apoptosis [100].
3.2 Evading Immune Responses
One of the hallmark abilities of cancer cells is their capacity to evade immune detection and destruction. TDEs contribute to this immune evasion by releasing immunosuppressive EVs that interact with immune cells in the TME [98, 100]. Many of these EVs contain Fas Ligand (FasL), which induces apoptosis in activated anti-tumor immune cells, such as T-cells, and reduces the activity of NK cells [98].
Studies have shown that TDEs can carry immune-modulating proteins such as PD-L1, which binds to PD-1 receptors on cytotoxic T-cells, blocking their activation and proliferation. For example, GBM-derived EVs expressing PD-L1 can directly inhibit T-cell function [101]. Additionally, EVs from breast cancer cells have been shown to suppress T-cell activation by delivering TGF-β, which modulates T-cell behavior through the TGF-β/Smad signaling pathway.
TDEs also influence the polarization of immune cells, such as macrophages. For example, EVs released by HCC cells have been shown to contain lncRNA TUC339, which regulates macrophage activation and induces M2 macrophage polarization. M2-like macrophages are known to support tumor growth by promoting immune suppression and tissue remodeling [100]. Furthermore, TDEs can drive the expansion of regulatory T cells (Tregs) and the differentiation of MDSCs, both of which suppress effector T-cell activation and contribute to immune suppression in the TME [100, 102].
3.3 Reprogramming Energy Metabolism
Cancer cells rely on altered energy metabolism to support rapid growth and proliferation, and EVs play a role in this metabolic reprogramming. TDEs can increase the production of metabolic acids, contributing to the acidification of the TME, which enhances the secretion and uptake of EVs [98]. For instance, EVs from adriamycin-resistant breast cancer cells contain high levels of glutathione S-transferase P1 (GSTP1), a metabolic enzyme that helps detoxify harmful chemicals and reprogram glucose metabolism in recipient cells [100]. Moreover, EVs from breast cancer cells can transfer miR-122 to non-tumor cells, promoting cancer progression by altering glucose metabolism. Another example involves miRNA-105, which is found in tumor-derived EVs from breast cancer cells and enhances glutamine and glucose metabolism in CAFs, providing energy to tumor cells. In nutrient-scarce environments, CAFs expel metabolic waste products such as lactate, which are then taken up by cancer cells to fuel oxidative phosphorylation, creating a metabolic symbiosis that supports tumor growth. Cancer cells stimulate the expression of HIF1a to support this relationship, creating “pseudo-hypoxia” conditions that then promote the release of exosomes in a calcium-dependent manner via MCT1, supporting glycolysis and facilitating tumor growth and survival [103].
3.4 Angiogenesis Formation
Angiogenesis, the formation of new blood vessels, is a critical process in tumor development, allowing tumors to grow beyond a few millimeters by ensuring a supply of oxygen and nutrients. EVs play a significant role in this process by delivering angiogenic factors to endothelial cells in the TME [104]. Studies have demonstrated that hypoxic tumor cells release exosomes that contribute to angiogenesis by carrying pro-angiogenic factors like tissue factor VIIa, which activates the ERK1/2 pathway and upregulates heparin-binding EGF-like growth factor [96]. Additionally, TDEs can contain Delta-like 4 (Dll4), a molecule that promotes angiogenesis by suppressing the Notch signaling pathway, which normally inhibits the development of new blood vessels. In HCC, exosomes carrying miR-210 have been shown to promote angiogenesis by inhibiting negative regulators such as SMAD4 and STAT6 [105].
Exosomal miRNAs also regulate cancer invasion and metastasis by altering gene expression in endothelial and immune cells, creating a premetastatic environment. For example, exosomal miR-200 has been shown to modify gene expression patterns, enhancing the metastatic potential of poorly metastatic cancer cells [96].
4 Role of Exosomes in the Progression of Solid Tumors
Exosomes are small vesicles that carry various cellular components, including proteins, nucleic acids, and lipids, and play a critical role in intercellular communication under both normal and pathological conditions [106]. Exosomes facilitate the transfer of miRNAs such as miR-21, miR-29a, miR-221, and miR-222, which reflect the molecular profiles of tumors. The cargo of exosomes varies depending on the cell type and origin, and recent studies have shown differences in the lipid composition of exosomes derived from cancer cells compared to those from non-cancer cells [10].
4.1 Brain Tumor
Exosomes play a pivotal role in brain tumors, especially GBM, where they promote cell proliferation and inhibit apoptosis. Proteomic analyses of GBM exosomes have revealed over 1000 proteins, including pro-angiogenic factors like interleukin-6 (IL-6), IL-8, and angiogenin, which contribute to hypoxia in brain endothelial cells and tumor malignancy [23, 107]. miR-21 and miR-451, found in GBM exosomes, are highly expressed in the cerebrospinal fluid (CSF) and plasma of GBM patients, making them useful biomarkers for diagnosing and monitoring tumor progression. miR-21 inhibits caspase activity, promoting cell survival, while miR-451 regulates proliferation and migration by suppressing the AMPK signaling pathway, which adapts to metabolic stress [106-109].
In neuroblastoma (NB), exosomes carry proteins involved in key biological processes like cell differentiation and proliferation. Exosomal miR-17-5p, secreted by MYCN-amplified NB cells, enhances the proliferation and migration of non-MYCN-amplified cells, highlighting the malignant role of exosomes in tumor progression [110, 111]. Additionally, exosomal miR-375 has been implicated in bone marrow metastasis in NB, as it promotes the differentiation of MSCs into osteogenic cells, creating a microenvironment conducive to tumor growth [112]. Targeting miR-375 could potentially reduce metastasis, making it a promising biomarker and therapeutic target for NB patients [112, 113].
4.2 Liver Tumor
In HCC, exosomal miRNAs and circular RNAs (circRNAs) play crucial roles in promoting tumor proliferation and immune evasion. miR-21 is carried by HCC-derived exosomes [114, 115]. It Transforms HSCs into CAFs by downregulating PTEN and activating the PDK1/AKT pathway. This leads to increased secretion of pro-angiogenic factors such as VEGF and TGF-β, supporting angiogenesis and tumor invasion [25, 116-118]. miR-665, another exosomal miRNA, promotes cellular proliferation through the MAPK/ERK pathway, enhancing metastatic potential [119]. Conversely, the downregulation of miR-100b and miR-125 in HCC exosomes activates the IGF2/AKT/mTOR pathway, essential for maintaining the stemness of HCC cells [120].
CircRNAs within HCC-derived exosomes also facilitate immune evasion by inducing T-cell exhaustion and promoting regulatory T cell (Treg) expansion. For instance, cirCCAR1, found in exosomes, inhibits CD8+ T-cell activity by upregulating PD-1 expression, leading to reduced cytotoxicity against HCC cells [19]. Additionally, circTMEM45A in HCC exosomes sponges miR-665, promoting IGF2 expression and cell proliferation by accelerating the G1/S cell cycle transition, contributing to tumor growth [121, 122].
Exosomes also modify the TME through EMT pathways. Circ_0004277 from HCC exosomes activates the EMT pathway by stimulating mesenchymal markers like N-cadherin, which facilitates HCC cell migration and the progression of normal cells into malignant ones [26]. Similarly, circ_0061395 enhances tumor proliferation by sponging miR-877-5p, leading to increased expression of the oncogene PIK3R3 [123, 124]. Circ_002136 derived from HCC cells Huh7 and HA22T cells increased proliferation, migration, and invasiveness of HCC cells through sponging of miR-19a-3p and downregulating RAB1A, which are members of Rab proteins needed for the trafficking of amino acids from the Golgi apparatus and endoplasmic reticulum, along with upregulation of the mTOR signaling pathway for cellular growth and migration [125].
4.3 Breast Cancer
Breast cancer-derived exosomes play a critical role in tumor progression by influencing stromal cells in the TME. Exosomal miR-135b-5p promotes the transition of MSCs into CAFs by inhibiting TXNIP, while miR-146a in exosomes converts normal fibroblasts into CAFs, which enhances metastasis in MCF-7 cells [126, 127]. Additionally, exosomal Survivin from breast cancer cells transforms fibroblasts into CAFs by upregulating superoxide dismutase 1 (SOD1), contributing to tumor growth [27, 128].
Exosomal miRNAs, including miR-21, miR-19b-3p, and miR-5100, also regulate the EMT, promoting metastasis [10, 129]. miR-1910-3p, encapsulated in breast cancer exosomes, enhances metastasis by activating the Wnt/β-catenin and NF-κB signaling pathways, while downregulating tumor suppressors like myotubularin-related protein 3 [28].
Long non-coding RNAs (lncRNAs) in exosomes also contribute to breast cancer progression [130, 131]. For example, lncRNA BCRT1, overexpressed in breast cancer exosomes, is associated with poor prognosis and promotes macrophage polarization to a tumor-supportive phenotype [132]. Additionally, Lnc GS1-600G8.5, carried by exosomes, facilitates brain metastasis by disrupting the BBB, while reduced expression of lncXIST has been linked to increased brain metastasis via EMT and c-Met signaling pathways [133].
5 Exosome-Based Therapeutics for Targeting Solid Tumors
Exosomes, due to their small size, biocompatibility, and ability to carry various molecules, have gained attention as potential tools in cancer therapy. Their ability to pass through biological barriers and be engineered for targeted delivery makes them promising candidates for drug delivery systems. Tumor-derived exosomal RNA can serve as biomarkers for cancer screening and diagnosis, while exosome-based therapies can initiate antitumor immune responses and improve drug delivery [134, 135].
5.1 Brain Cancer
The BBB poses a significant challenge for the effective treatment of brain cancers such as GBM. Exosomes have emerged as potential nanocarriers for anticancer therapies due to their ability to cross the BBB. Their stability, low immunogenicity, and high loading capacity make them ideal candidates for delivering drugs or therapeutic molecules directly to the tumor site.
One promising approach uses dendritic cell-derived exosomes, which have demonstrated immune-related antitumor activity in GBM mouse models [136]. Additionally, exosomes from MSCs have shown potential in delivering therapeutic miRNAs to glioma cells. For example, miR-199a delivered via MSC-derived exosomes inhibits glioma cell proliferation and migration by downregulating AGAP2 expression, leading to increased apoptosis in glioma cells [137].
Another promising strategy involves the use of exosomal miR-151a, which has been linked to overcoming temozolomide (TMZ) resistance in GBM. Chemo-resistant GBM cells have lower levels of miR-151a in their exosomes, contributing to resistance. Restoring miR-151a in exosomes could reverse this resistance, making it a potential therapeutic target for refractory GBM [138-140].
Exosomes also offer potential for drug delivery. Studies in zebrafish and mouse models have shown that exosomes derived from glioma and brain endothelial cells can effectively deliver chemotherapeutic agents such as paclitaxel (PTX) and doxorubicin (DXR) across the BBB, reducing tumor progression and overcoming chemoresistance [24, 141]. Additionally, exosomes containing microRNA-29a-3p have shown promise in suppressing angiogenesis and migration in resistant gliomas, offering a novel anti-angiogenic therapeutic approach [8].
5.2 Liver Cancer
Hepatocellular carcinoma is commonly treated with surgical resection, ablation, or targeted drug therapies. While conventional therapies such as sorafenib and regorafenib are widely used, exosome-based therapies are emerging as novel strategies for enhancing treatment outcomes [142]. Dendritic cell-derived exosomes (DEX), for instance, have shown promise in activating T cells and inducing antitumor responses in HCC by upregulating IFN-γ and IL-2 while downregulating inhibitory cytokines like TGF-β and IL-10 [143, 144].
NK cell-derived exosomes also show potential in inducing apoptosis in HCC cells through perforin and granzyme B pathways, leading to the activation of caspases and promoting cell death [145, 146]. Moreover, engineered exosomes carrying miR-654-5p have been shown to induce ferroptosis in HCC cells by inhibiting HSPB1, enhancing the efficacy of sorafenib, a drug that is often hindered by resistance due to HSPB1 overexpression [147]. Combination therapies using exosomes have demonstrated enhanced therapeutic effects. For instance, dendritic cell-derived exosomes combined with microwave ablation resulted in reduced tumor size by decreasing Tregs and increasing CD8+ T cells, a more effective outcome than either treatment alone [148, 149]. The combination of exosomes derived from dendritic cells, along with P47 (peptide targeting the HCC cells), HMGN1 (recruiting the DC), and AFP212-A2 (exosomal anchors), on injection, suppressed tumor growth and increased the immune responses through immune-stimulatory CD8+ effector T-cell recruitment and IFN-g along with a decrease in immunosuppressive factors such as TGF-b and IL-10 [150]. This approach highlights the potential for exosome-based combination therapies in improving immune responses and reducing tumor progression in HCC.
5.3 Breast Cancer
Breast cancer-derived exosomes (TDEs) have garnered significant interest for their potential in diagnosis and therapy. Engineered exosomes, enriched with miRNAs, have been explored as therapeutic alternatives. For example, exosomal miR-134 has been shown to inhibit the invasion and migration of breast cancer cells while increasing their sensitivity to anti-HSP90 inhibitors like PU-H71 [151]. Another miRNA, miR-503, inhibits breast cancer cell invasion by targeting CCND3 and CCND2, both of which are involved in cell cycle regulation, as shown in Figure 4 [152, 153].
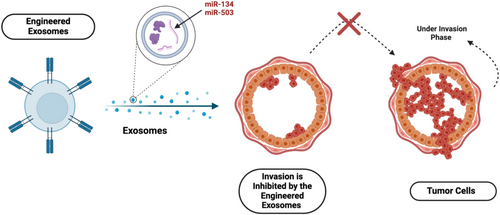
Exosomes also play a role in modulating the TME. Studies have shown that exosomes carrying miR-135b-5p can induce the transition of MSCs into CAFs, promoting tumor growth and metastasis [104]. Additionally, exosomal long non-coding RNA (lncRNA) BCRT1, overexpressed in breast cancer cells, promotes macrophage polarization and enhances tumor progression [154-156]. The potential of exosomes in breast cancer treatment is evident in their ability to carry therapeutic molecules and modulate the TME. However, further research is needed to develop reliable protocols for isolating, characterizing, and utilizing exosomes in clinical applications.
6 Clinical Applications of Exosome Biomarkers
Traditional cancer diagnostic methods, primarily imaging and invasive biopsies, are costly, often uncomfortable, and typically detect cancer at later stages, particularly in the absence of routine screening. Late-stage diagnosis diminishes treatment efficacy and reduces survival rates [157, 158]. This has prompted a shift towards less invasive, cost-effective methods for early detection and continuous monitoring. Exosomes, cell-derived EVs found in biofluids such as blood, plasma, serum, urine, and saliva, are emerging as valuable tools in this area due to their molecular contents that reflect cellular states [159-161].
Several isolation techniques for exosomes have been developed, including size-exclusion chromatography (SEC) [162-164], ultracentrifugation, and combined approaches like ultrafiltration with SEC [165]. Recently, protocols utilizing ultracentrifugation paired with tangential flow filtration (TFF) and SEC have demonstrated higher efficiency, specificity, and reproducibility in isolating exosomes from serum [166].
Exosomes are rich in stable proteins and microRNAs, including cancer-specific molecules that provide insight into tumor biology. Liquid biopsies analyzing these exosomal contents offer non-invasive diagnostic and monitoring capabilities for various cancers. For instance, in brain cancers, exosomes can cross the BBB and carry glioma markers like IDH1G395A, providing a method to monitor tumor progression through blood samples [167]. Additionally, EGFRvIII, an oncogenic marker frequently upregulated in high-grade gliomas, can be detected in serum-derived exosomes with a sensitivity of 81.58% and specificity of 79.31% [168]. In HCC, exosomal miR-21 is associated with tumor stage, cirrhosis, and chemotherapy resistance, making it a sensitive biomarker for HCC diagnosis compared to serum miR-21 (Wang et al. [169], Cao et al. [170]). Breast cancer diagnostics also benefit from exosomal miRNAs; for example, miR-939, linked to poor prognosis, is overexpressed in basal-like and triple-negative breast cancer subtypes [171]. Exosomal biomarkers hold promising clinical applications for non-invasive cancer diagnosis and monitoring, supporting early detection and providing crucial insights into tumor progression and therapeutic responses.
7 Conclusion
Exosomes have emerged as key contributors to the progression of solid cancers by facilitating cell communication and shaping the TME. In brain tumors like GBM and NB, exosomes influence disease progression by mediating interactions within the TME. In liver cancer, the regulation of microRNAs and circRNAs via exosomes is pivotal for tumor growth and metastasis. Similarly, in breast cancer, exosomes play dual roles, contributing to both immunosuppression and metastasis. A deeper understanding of EV signaling in cancer is essential for addressing growth resistance and informing therapeutic strategies. Research has demonstrated that the cargo of EVs is highly dynamic, particularly in physiologically relevant tumor models, and evolves throughout different stages of cancer progression. However, most experimental evidence has been derived from in vitro and animal models, and there has been limited progress in clinical research. Future studies should focus on pathophysiological models in living organisms with tumors, rather than relying solely on interactions with single cells. The potential clinical implications of understanding EV dynamics are significant. A better grasp of EV signaling networks could help predict tumor progression and enable the development of innovative strategies to restore control over these networks in cancer patients. By emphasizing the clinical relevance of this research, we highlight the direct impact it can have on improving cancer treatment and patient outcomes.
Author Contributions
Nada Aakel: validation (equal), writing – original draft (equal). Rawdhah Mohammed: writing – original draft (equal). Aseela Fathima: writing – original draft (equal). Rabia Kerzabi: writing – original draft (equal). Atiyeh Abdallah: validation (equal), writing – review and editing (equal). Wisam Nabeel Ibrahim: conceptualization (lead), project administration (lead), supervision (lead), writing – review and editing (lead).
Acknowledgments
The publication of this article was supported by Qatar National Library. Qatar University Open Access publishing facilitated by the Qatar National Library, as part of the Wiley Qatar National Library agreement.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
This review article does not include any primary data. All data discussed and analyzed in this review are from previously published studies, which are cited and available through publicly accessible databases and publications. No new data were generated for this study.