Significance of Mutation Spots and Concurrent Gene Mutations on Prognosis and Clinical Outcomes in Myelodysplastic Syndromes With SF3B1 Mutation
Funding: This research was funded by the General Program of Shanghai Science and Technology Commission (22ZR1447700).
ABSTRACT
Purpose
To investigate the clinical characteristics and prognosis of mutation spots and concomitant gene mutations in myelodysplastic syndromes (MDS) with SF3B1 mutation (SF3B1mut).
Patients and Methods
Patients diagnosed with MDS at Shanghai Jiao Tong University School of Medicine Affiliated Sixth People's Hospital from October 2008 to November 2023 were enrolled in this study. SF3B1mut was identified by next-generation sequencing (NGS).
Results
One hundred and seven (8.7%) cases harbored the SF3B1 mutation. The most frequent SF3B1mut, noted in 47.66% of all patients, was the hotspot K700E. K666 and R625 were observed in 24.30% and 9.35%, respectively. Two less frequent mutation subtypes accounted for 5.61% of H662 and 4.67% of E622. Patients with the K666 mutation showed more severe thrombocytopenia (p = 0.032), significantly lower NK cell percentage (p = 0.001), and the Th1/Th2 ratio (p = 0.018) in the bone marrow (BM). The overall survival (OS) in patients with E622 and H662 mutations was significantly longer than that of patients with the R625 mutation (p = 0.045) and the K666 mutation (p = 0.010). Multi-variance analysis showed the SF3B1 mutation involving the K666 hotspot independently predicted overall survival in MDS (HR 2.094, p = 0.050). Notably, most (11/13, 84.6%) of concomitant TP53 mutations were mono-hit, which did not affect the survival of patients in our cohort.
Conclusions
SF3B1mut patients with specific mutation spots and concomitant gene mutations showed distinct clinical features and prognosis. Consequently, a comprehensive study of specific subtypes is of great significance for improving the prognosis of patients with SF3B1 mutations.
1 Introduction
Myelodysplastic syndromes (MDS) are a group of clonal malignant diseases that originate from hematopoietic stem cells, which are characterized by ineffective hematopoiesis and cytopenia, with an increased risk of transforming into acute myeloid leukemia (AML) [1]. Genetic mutations are closely related to the pathogenesis of MDS, the most common of which include genes related to RNA splicing [2]. SF3B1 is one of the most frequently mutated splicing genes in MDS, mutations of which may cause the abnormal gene splicing of hemoglobin synthesis and iron metabolism, and then result in the dysynthesis of hemoglobin and the formation of ringed sideroblasts (RS) [3-5]. The most common clinical characteristic is anemia. Multiple researches have shown that the SF3B1 mutation (SF3B1mut) is associated with a favorable prognosis and a lower possibility of transforming to AML. The International Working Group for the Prognosis of MDS has proposed SF3B1mut MDS as a distinct disease subtype [1]. In the 5th edition of the classification of hematolymphoid tumors (WHO-HAEM5), MDS with low blasts and SF3B1 mutation was defined as a new subtype [6].
K700E is the most frequent mutation spot in SF3B1mut MDS. Previous research has discovered that patients with the K700E mutation show a favorable prognosis, while those with the K666 mutation are associated with a negative prognosis. However, information about the clinical features and prognosis of non-K700E and non-K666 mutations in SF3B1 mutant MDS is scarce. SF3B1mut MDS patients with complex mutational status (at least two associated mutations) are associated with a poor prognosis. Specific genes are associated with the unfavorable prognosis in MDS. More than 40% of patients with MDS have at least two mutations, and the co-occurrence of particular genes may change the effect on the prognosis of a single mutation. IPSS-M includes somatic mutations in 31 genes to provide a risk score. Previous studies have divided SF3B1mut MDS into three independent groups: SF3B15q, SF3B1α, and SF3B1β. The favorable outcomes are only confined to the SF3B1α group without mutations in BCOR, BCORL1, RUNX1, NRAS, STAG2, and SRSF2, as well as del (5q) [7]. However, little is known about the impact of TP53 concurrent mutations on the prognosis of SF3B1mut MDS. In addition, the pathogenesis of MDS involves various factors, and it is not yet fully elucidated. With the exception of molecular genetics and cytogenetics, immunity has been confirmed to play a role in the pathogenesis of MDS. According to previous findings, the activation of inflammatory immune signaling is seen in SF3B1mut MDS. However, the immune characteristics of mutational subtypes in SF3B1mut MDS have not been explored. In the current study, the clinical features, immune cells in bone marrow (BM), and prognosis of patients with different SF3B1 mutation spots will be explored. Furthermore, concomitant gene mutations, including the TP53 mutation, will also be analyzed in our cohort for their prognostic value in SF3B1mut patients.
2 Materials and Methods
2.1 Patients
Patients diagnosed with MDS according to WHO 2016 [8] at Shanghai Jiao Tong University School of Medicine Affiliated Sixth People's Hospital from October 2008 to November 2023 who harbored the SF3B1 mutation identified by next-generation sequencing (NGS) were enrolled in this study. Clinical characteristics including age, sex, hemoglobin, platelet, absolute neutrophil count in the peripheral blood, blasts and RS in bone marrow (BM), cytogenetics, serum ferritin, and serum erythropoietin level were analyzed in this study. Follow-up time started from the date of the diagnosis of MDS and ended on June 1, 2024. Overall survival (OS) was defined as the date of diagnosis to the date of death, end of follow-up, or loss to follow-up. Leukemia-free survival (LFS) was defined as the time from disease diagnosis to progression to leukemia or death. The study was approved by the Ethics Committee of Shanghai Sixth People's Hospital Affiliated to Shanghai Jiao Tong University School of Medicine and was in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent from all patients was obtained.
2.2 Targeted Gene Sequencing
Thirty-eight genes, including ASXL1, ANKRD11, BCOR, CALR, CBL, CEBPA, DNMT3A, ETV6, EZH2, FLT3, GATA2, IDH1, IDH2, ITIH3, JAK2, KIF20B, KIT, KRAS, MPL, NF1, NPM1, NRAS, PHF6, PTPN11, PTPRD, ROBO1, ROBO2, RUNX1, SETBP1, SF3B1, SRSF2, STAG2, TET2, TP53, U2AF1, UPF3A, WT1, and ZRSR2 were examined for mutations by MiSeq sequencing (Illumina, San Diego, CA, USA) in gDNA from BM mononuclear cells of patients. Detailed operating steps were described in our previous studies [9]. [Correction added on July 28, 2025 after first online publication. The term ‘cDNA’ has been replaced with ‘gDNA’ in the previous sentence.]
2.3 Flow Cytometry Analysis
T cell subsets and NK cells in BM were detected with Coulter Epics-XL (Beckman Coulter) and analyzed by System II Software (Beckman Coulter). The following antibodies were used in this study: PC5-labeled anti-CD3, ECD-labeled anti-CD8, fluorescein isothiocyanate (FITC)-labeled anti-human IFN-γ, and phycoerythrin (PE)-labeled anti-human IL-4 (Beckman Coulter). CD3+/CD8− cells were defined as CD4+ cells. Th and Tc subsets were defined as Th1 (CD8−/INF-γ+), Th2 (CD8−/IL-4+), Tc1 (CD8+/INF-γ+), and Tc2 (CD8+/IL-4+). Detailed operating steps were described in our previous studies [10].
2.4 Enzyme-Linked Immunosorbent Assay (ELISA)
The levels of serum erythropoietin and serum ferritin were measured by ELISA. The normal reference value of the level of serum erythropoietin is from 4.3 mIU/mL to 29.0 mIU/mL, and the normal reference value of the level of serum ferritin is from 30.0 ng/mL to 400.0 ng/mL.
2.5 Statistical Analysis
SPSS version 27.0 and GraphPad Prism 10.0 were used for statistical analysis. Continuous variables that followed the normal distribution were represented by the mean, and continuous variables that did not conform to the normal distribution were represented by the quartile. The differences between continuous variables were compared by the independent-samples t test, one-way analysis of variance, Mann–Whitney U test, and Kruskal–Wallis test. A chi-square test was used to compare the differences between the categorical variables. The Kaplan–Meier curve was used for survival analysis. Log-rank was used to test the difference in OS. Based on univariate analysis, variables with statistically significant differences were analyzed by Cox multivariate analysis. p < 0.05 was considered statistically significant.
3 Results
3.1 Clinical Characteristics of Mutation Spots in SF3B1mut MDS
A total of 1226 MDS patients were enrolled in this study, and 107 (8.7%) cases harbored SF3B1 mutations. The median variant allele frequency (VAF) of the SF3B1 gene was 39.2% (range: 2.5%–59.0%). There were 65 males and 42 females, with a median age of 66 years (range: 20–89 years). According to the 2016 WHO categories, 4.9% of patients were diagnosed as MDS with single lineage dysplasia (MDS-SLD), 12.6% of patients were diagnosed as MDS with multilineage dysplasia (MDS-MLD), 57.3% of patients were diagnosed as MDS with ring sideroblasts (MDS-RS), 14.6% of patients were diagnosed as MDS with excess blasts-1 (MDS-EB-1), 8.7% of patients were diagnosed as MDS with excess blasts-2 (MDS-EB-2), 1.0% of patients were diagnosed as MDS with isolated deletions of the long arm of chromosome 5 (del [5q]), and 1.0% of patients were diagnosed as unclassifiable MDS (MDS-U). Cytogenetic analysis showed that 43.9% of cases had the normal karyotype, 4.7% of cases had del (5q), 7.5% of cases had deletions of the long arm of chromosome 20 (del [20q]), 6.5% of cases had trisomy 8, 6.5% of cases had complex karyotypes, 2.8% of cases had abnormalities of chromosome 3, and 0.9% of cases had loss of chromosome 7. According to the revised International Prognostic Scoring System (IPSS-R), patients were classified as very low-risk group (4.3%), low-risk group (33.7%), intermediate-risk group (40.2%), high-risk group (9.8%), and very-high risk group (12.0%). Among IPSS-R categories, 78.2% of cases were relatively low-risk groups, including very-low risk, low-risk, and intermediate-risk group, whereas 21.8% of cases were relatively high-risk groups, including high-risk and very-high risk group. Among all patients, only 17.5% of patients progressed to AML.
Further, we compared the clinical features of different mutation spots in SF3B1mut patients. Fifty-one of 107 cases (47.66%) had the K700E mutation with the median VAF of 39.65%, 26 cases (24.30%) had the K666 mutation with the median VAF of 41.35%, 10 cases (9.35%) had the R625 mutation with the median VAF of 37.10%, 6 cases (5.61%) had the H662 mutation with the median VAF of 21.00%, and 5 cases (4.67%) had the E622 mutation with the median VAF of 35.00% (Figure 1b and Table 1). Patients with E622 and H662 mutations showed lower VAF compared to those with non-E622 and non-H662 mutations (median, 22.35% vs. 40.20%, p = 0.059), and SF3B1H662 patients showed significantly lower VAF (median, 21.00% vs. 40.00%, p = 0.034, Figure S1). Two patients had double mutation spots. One case had both K700E and E622 mutations, and the other had K700E and R939H mutations. The remaining hotspots were E592K, A744P, G740E, L638F, G751V, and A1282E (7/107, 6.54%) (Figure 1b). In our cohort, the median hemoglobin was 71.8 g/L in patients with the SF3B1 mutation. Of the total patients, 51.4% showed moderate anemia, 28.0% showed severe anemia, and 11.2% showed mild anemia. The median hemoglobin was 76.0 g/L in patients with E622 and H662 mutations compared to those without these mutations (median, 76.0 g/L vs. 68.5 g/L, p = 0.059). SF3B1K666 patients showed significantly lower platelets compared to those with non-K666 mutations (median, 78.0 × 109/L vs. 127.5 × 109/L, p = 0.032, Figure 1d), whereas SF3B1R625 patients showed significantly higher platelets compared to those with non-R625 mutations (median, 189.0 × 109/L vs. 111.5 × 109/L, p = 0.020, Figure 1d). The platelet count had no statistical significance in patients with E622 and H662 mutations compared to those with other subtype mutations. There was no discernible difference in the absolute neutrophil count within the five groups with varying subtype mutations (Figure 1e). The percentage of BM blasts was lower in SF3B1mut patients with E622 and H662 hotspots, although without statistical significance (median, 0.50% in E622 and H662 vs. 1.35% in non-E622 and non-H662, p = 0.060). In our cohort, 75.0% of patients with the SF3B1 mutation had a RS percentage higher than 5%. Of these patients, 90.5% had a percentage of RS greater than 15%. Of SF3B1mut patients with the K700E mutation, 88.6% had an RS percentage higher than 5%, compared to 71.4% of SF3B1mut patients with the R625 mutation, 66.7% of SF3B1mut patients with E622, 63.2% of SF3B1mut patients with the K666 mutation, and 25.0% of SF3B1mut patients with the H662 mutation. The percentage of RS was significantly lower in SF3B1mut patients with the K666 mutation compared to those with non-K666 mutations (median, 16.8% vs. 36.0%, p = 0.021), while significantly higher in SF3B1mut patients with the K700E mutation compared to those with non-K700E mutations (median, 39.0% vs. 18.0%, p = 0.013). In our cohort, 30.8% of SF3B1mut patients had a serum ferritin level above 1000 ng/mL. The median serum ferritin was significantly lower in SF3B1mut patients with the K666 mutation compared to those with non-K666 mutations (median, 497.1 ng/mL vs. 874.0 ng/mL, p = 0.022). A total of 47.7% of patients had serum erythropoietin above 500 mIU/mL.
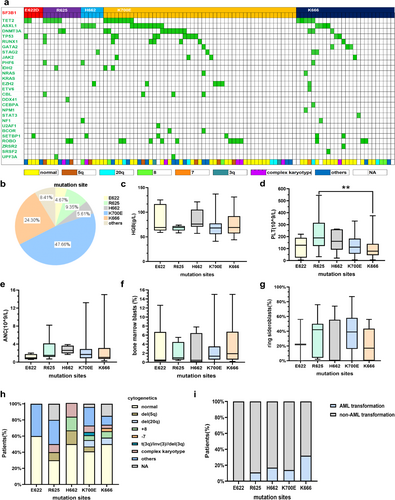
| Characteristics | E622, n = 5 | R625, n = 10 | H662, n = 6 | K700E, n = 51 | K666, n = 26 | p |
|---|---|---|---|---|---|---|
| HGB | ||||||
| Median (g/L) | 69.0 | 69.0 | 76.0 | 68.0 | 69.0 | 0.342 |
| Range (g/L) | 63.0 to 116.5 | 63.0 to 72.0 | 70.5 to 105.3 | 55.5 to 77.3 | 57.0 to 92.0 | |
| PLT | ||||||
| Median (109/L) | 128.0 | 189.0 | 160.5 | 112.0 | 78.0 | 0.050 |
| Range (109/L) | 23.8 to 190.0 | 122.0 to 315.5 | 91.3 to 260.8 | 56.5 to 183.3 | 45.0 to 140.0 | |
| ANC | ||||||
| Median (109/L) | 0.95 | 1.40 | 2.60 | 1.70 | 1.05 | 0.208 |
| Range (109/L) | 0.65 to 1.78 | 0.90 to 4.15 | 1.95 to 3.65 | 0.88 to 2.90 | 0.70 to 3.13 | |
| RS | ||||||
| Median (%) | 22.0 | 42.0 | 0.0 | 39.0 | 16.8 | 0.070 |
| Range (%) | 0.0 to — | 4.0 to 50.0 | 0.0 to 55.5 | 16.0 to 58.0 | 0.0 to 43.5 | |
| BM blasts | ||||||
| Median (%) | 0.50 | 1.00 | 0.45 | 1.35 | 1.90 | 0.404 |
| Range (%) | 0.20 to 6.70 | 0.60 to 4.47 | 0.05 to 6.45 | 0.60 to 3.50 | 0.60 to 6.75 | |
| CD4 | ||||||
| Median (%) | 29.58 | 31.34 | 37.33 | 31.71 | 33.98 | 0.595 |
| Range (%) | 29.04 to 38.25 | 23.95 to 37.02 | 32.09 to 41.00 | 25.89 to 37.28 | 26.58 to 37.54 | |
| CD8 | ||||||
| Mean (%) | 37.08 | 36.12 | 33.68 | 37.97 | 38.54 | 0.948 |
| Range (%) | 16.00 to 51.00 | 24.80 to 52.30 | 25.00 to 42.83 | 13.47 to 75.48 | 10.00 to 65.69 | |
| CD4/CD8 | ||||||
| Median | 0.86 | 0.77 | 1.14 | 0.92 | 0.83 | 0.721 |
| Range | 0.66 to 1.57 | 0.65 to 1.31 | 0.81 to 1.53 | 0.61 to 1.12 | 0.67 to 1.05 | |
| Th1 | ||||||
| Median (%) | 10.00 | 22.40 | 26.79 | 22.42 | 17.21 | 0.862 |
| Range (%) | 3.75 to 43.18 | 12.19 to 37.99 | 7.50 to 32.25 | 13.17 to 33.70 | 12.69 to 34.07 | |
| Th1/Th2 | ||||||
| Median | 49.0 | 36.4 | 49.1 | 44.8 | 19.0 | 0.162 |
| Range | 21.5 to 103.9 | 13.6 to 99.8 | 35.0 to 134.2 | 21.9 to 94.1 | 11.8 to 56.4 | |
| Tc1 | ||||||
| Mean (%) | 39.42 | 45.10 | 40.77 | 48.55 | 56.51 | 0.510 |
| Range (%) | 4.60 to 76.10 | 16.42 to 70.00 | 13.50 to 63.78 | 2.20 to 88.19 | 23.00 to 93.95 | |
| Tc1/Tc2 | ||||||
| Median | 149.5 | 74.9 | 151.6 | 130.0 | 123.6 | 0.560 |
| Range | 102.5 to 242.1 | 37.3 to 150.9 | 74.0 to 365.0 | 75.5 to 272.0 | 72.9 to 218.5 | |
| NK | ||||||
| Median (%) | 18.45 | 24.10 | 14.00 | 18.30 | 8.41 | 0.032 |
| Range (%) | 8.75 to 40.23 | 14.58 to 35.17 | 9.89 to 20.82 | 9.54 to 24.32 | 3.66 to 20.56 | |
| VAF | ||||||
| Median (%) | 35.00 | 37.10 | 21.00 | 39.65 | 41.35 | 0.299 |
| Range (%) | 12.10 to 51.30 | 32.68 to 44.83 | 16.25 to 34.10 | 29.90 to 44.85 | 28.15 to 47.15 | |
| Number of mutant genes | ||||||
| 1, n (%) | 1 (20.0) | 0 (0.0) | 1 (16.7) | 7 (13.7) | 6 (23.1) | 0.468 |
| 2, n (%) | 3 (60.0) | 3 (30.0) | 2 (33.3) | 17 (33.3) | 5 (19.2) | |
| ≥ 3, n (%) | 1 (20.0) | 7 (70.0) | 3 (50.0) | 27 (52.9) | 15 (57.7) | |
| TET2 | ||||||
| TET2, n (%) | 2 (40.0) | 5 (50.0) | 0 (0.0) | 8 (15.7) | 5 (19.2) | 0.068 |
| Non-TET2, n (%) | 3 (60.0) | 5 (50.0) | 6 (100.0) | 43 (84.3) | 21 (80.8) | |
| ASXL1 | ||||||
| ASXL1, n (%) | 0 (0.0) | 0 (0.0) | 4 (66.7) | 9 (17.6) | 4 (15.4) | 0.027 |
| Non-ASXL1, n (%) | 5 (100.0) | 10 (100.0) | 2 (33.3) | 42 (82.4) | 22 (84.6) | |
| TP53 | ||||||
| TP53, n (%) | 1 (20.0) | 1 (10.0) | 0 (0.0) | 9 (17.6) | 2 (7.7) | 0.653 |
| Non-TP53, n (%) | 4 (80.0) | 9 (90.0) | 6 (100.0) | 42 (82.4) | 24 (92.3) | |
| DNMT3A | ||||||
| DNMT3A, n (%) | 0 (0.0) | 2 (20.0) | 0 (0.0) | 7 (13.7) | 3 (11.5) | 0.880 |
| Non- DNMT3A, n (%) | 5 (100.0) | 8 (80.0) | 6 (100.0) | 44 (86.3) | 23 (88.5) | |
| RUNX1 | ||||||
| RUNX1, n (%) | 0 (0.0) | 1 (10.0) | 0 (0.0) | 5 (9.8) | 1 (3.8) | 0.839 |
| Non- RUNX1, n (%) | 5 (100.0) | 9 (90.0) | 6 (100.0) | 46 (90.2) | 25 (96.2) | |
| STAG2 | ||||||
| STAG2, n (%) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 1 (2.0) | 2 (7.7) | 0.270 |
| Non- STAG2, n (%) | 5 (100.0) | 10 (100.0) | 5 (83.3) | 50 (98.0) | 24 (92.3) | |
| EZH2 | ||||||
| EZH2, n (%) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 3 (5.9) | 1 (3.8) | 0.615 |
| Non-EZH2, n (%) | 5 (100.0) | 10 (100.0) | 5 (83.3) | 48 (94.1) | 25 (96.2) | |
| Status | ||||||
| AML transformation, n (%) | 0 (0.0) | 1 (11.1) | 1 (16.7) | 7 (14.3) | 8 (32.0) | 0.324 |
| Non-AML transformation, n (%) | 5 (100.0) | 8 (88.9) | 5 (83.3) | 42 (85.7) | 17 (68.0) | |
- Note: Bold part indicates meaningful statistical results.
- Abbreviations: AML, acute myeloid leukemia; ANC, absolute neutrophil count; BM, bone marrow; HGB, hemoglobin; PLT, platelet; RS, ringed sideroblasts; VAF, variant allele frequency.
The serum erythropoietin did not reach the statistical difference between patients with distinct subtype mutations (Table S1). Among the IPSS-R categories, SF3B1K666 patients showed a higher percentage in the higher risk group compared to those with non-K666 mutations (36.8% vs. 20.3%, p = 0.024). Conversely, almost all of the patients with the E622 mutation were in the lower risk group. Regarding the 2016 WHO categories, SF3B1mut patients were both enriched in WHO categories with MDS-RS. MDS with isolated del (5q) was only found in patients with the R625 mutation (Table S1). Further analysis of the above characteristics showed no differences in the subgroup of patients with a normal karyotype (Table S2).
3.2 Immune Characteristics of Mutation Spots in SF3B1mut MDS
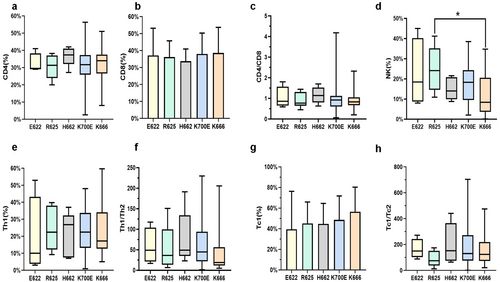
The analysis of immune characteristics of SF3B1mut patients mainly included CD4+ T cells, CD8+ T cells, CD4/CD8 ratio, Th1 cells, Th1/Th2 ratio, Tc1 cells, Tc1/Tc2 ratio, and NK cells in the BM. SF3B1R625 patients showed a significantly higher BM NK cell percentage (median: 24.10% vs. 14.67%, p = 0.025) compared to those with non-R625 mutations, whereas SF3B1K666 patients showed a significantly lower BM NK cell percentage compared to those with non-K666 mutations (median, 8.41% vs. 18.59%, p = 0.001). BM NK cell percentage was significantly higher in SF3B1mut patients with the R625 mutation compared to those with the K666 mutation (median, 24.10% vs. 8.41%, p = 0.028, Figure 2d). In addition, SF3B1mut patients with K666 mutations showed a significantly lower Th1/Th2 ratio compared to those with non-K666 mutations (median: 19.0% vs. 45.6%, p = 0.018, Figure 2f).
3.3 Concomitant Gene Mutations and Cytogenetic Changes in SF3B1mut MDS
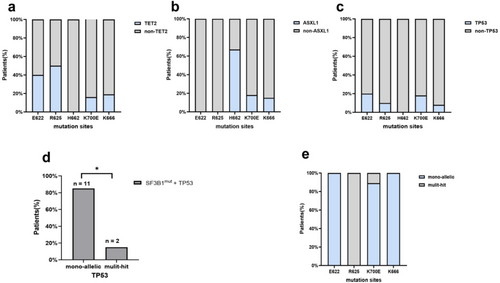
The most frequently mutant genes co-occurring with SF3B1 included TET2 (20.6%).
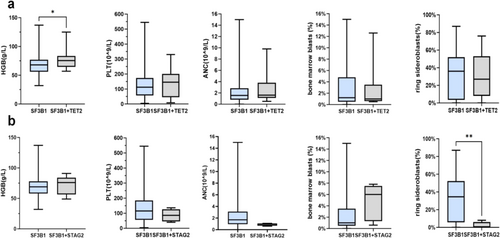
ASXL1 (19.6%), TP53 (12.1%), DNMT3A (11.2%), RUNX1 (6.5%), EZH2 (4.7%), and STAG2 (4.7%). TP53 mutations were divided into mono-hit and multi-hit according to the WHO 2022 classification for MDS. In our cohort of 107 cases with SF3B1 mutations, TP53 mutations were detected in 13 (12.1%) patients. Notably, most of the mutations in TP53 (11/13, 84.6%) were mono-hit (Figure 3d). In SF3B1mut patients with the H662 hotspot, ASXL1 (4/6, 66.7%) was the most frequently co-mutated gene, whereas TET2, TP53, DNMT3A, and RUNX1 mutations were not observed (Figure 3b). TET2 (5/10, 50%) was the most frequently co-mutant gene in patients with the R625 mutation, whereas ASXL1, STAG2, and EZH2 mutations were not observed (Figure 3a). Further analysis showed TET2 was also the common co-mutant gene in the subgroup of SF3B1R625 patients with normal karyotype (Table S2). Three patients had double-splicing gene mutations, which were the co-occurrence of the SF3B1 and U2AF1 mutation, the SF3B1 mutation and ZRSR2 mutation, and the SF3B1 mutation and SRSF2 mutation. Interestingly, the percentage of RS was significantly lower in SF3B1mut MDS patients with STAG2 mutation (median, 0.0% vs. 34.5%, p = 0.010, Figure 4b). Of patients with the co-occurrence of the SF3B1 mutation with the STAG2 mutation, 75% showed no percentage of RS. However, there were no differences in VAF between patients with or without specific co-mutations (Figure S4).
Cytogenetic analysis showed 43.9% of patients with the SF3B1 mutation had the normal karyotype, which had no statistical difference in SF3B1mut patients with specific mutation spots. The t (3q) and inv (3q) were observed in two SF3B1mut patients with the K700E mutation. In addition, t (3q) was also observed in a patient with K700E and E622 double mutation spots. A patient with the K666 mutation had a loss of chromosome 7 (Table S1). Of SF3B1mut patients, 6.5% had the complex karyotype. Further analysis showed no patients had the complex karyotype in SF3B1mut patients with the E622 mutation (Figure 1h). In SF3B1mut patients with the K666 mutation, the percentage of complex karyotype was significantly lower compared to the percentage of normal karyotype (3.8% vs. 50.0%, p = 0.001).
3.4 Prognosis of Patients With SF3B1mut MDS
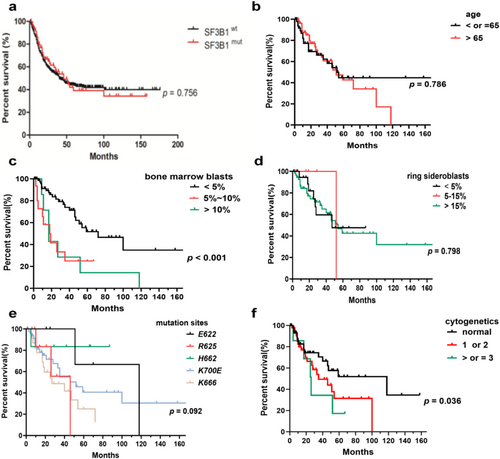
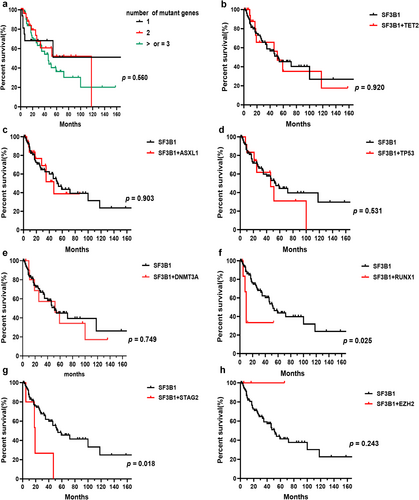
We analyzed the prognostic impact of gene mutations and other risk factors on OS in SF3B1mut MDS. The median OS in SF3B1mut patients showed no statistical difference compared to SF3B1wt patients (Figure 5a). The median OS of patients between the ages of under 65 and over 65 was similar (Figure 5b). The median OS of patients with BM blasts less than 5% was 72 months, which was longer than those with BM blasts more than 5% (p = 0.0004, Figure 5c). The median OS of patients with RS below 5%, between 5% and 15%, and above 15% was 47, 52, and 54 months, respectively, which showed no statistical difference (Figure 5d). Patients with E622 and H662 mutations had longer OS than those with other subtype mutations, which was significantly longer than patients with the R625 mutation (median OS, 118 months in E622 and H662 vs. 46 months in R625, p = 0.045) and the K666 mutation (median OS, 118 months in E622 and H662 vs. 27 months in K666, p = 0.010). The SF3B1-K666 mutation showed an inferior impact on the prognosis of patients compared to non-K666 mutations (median OS, 27 months vs. 52 months, p = 0.043). The prognostic impact of karyotypes on OS showed statistical significance (median OS, 118 months for patients with normal karyotypes vs. 35 months for patients with 1 or 2 abnormal karyotypes vs. 26 months for patients with complex karyotypes, p = 0.036, Figure 5f). Of SF3B1mut patients with the K666 mutation, 32.0% progressed to AML, compared to 16.7% of SF3B1mut MDS patients with the H662 mutation, 14.3% of SF3B1mut patients with K700E, and 11.1% of SF3B1mut patients with the R625 mutation. No patients with the E622 mutation progressed to AML (Table 1). Within all SF3B1mut patients, the number of mutant genes showed no significant impact on OS (Figure 6a). Compared to SF3B1mut with non-RUNX1 and non-STAG2 co-mutations, the median OS of patients with RUNX1 (median OS, 10 months vs. 52 months, p = 0.025, Figure 6f) and STAG2 co-mutations (median OS, 19 months vs. 54 months, p = 0.018, Figure 6 g) was worse. The co-mutations of TET2, ASXL1, TP53, DNMT3A, and EZH2 showed no discernible effects on OS (Figure 6b–e,h). Within a single-variable survival analysis, the BM blasts (HR 1.912, p < 0.001), cellular genetics (HR 1.627, p = 0.039), K666 mutation (HR 2.037, p = 0.030), RUNX1 co-mutation (HR 3.106, p = 0.034), and STAG2 co-mutation (HR 3.262, p = 0.027) were unfavorable factors of prognosis (Table 2). In multivariate survival analysis, the BM blasts (HR 1.661, p = 0.017), K666 mutation (HR 2.094, p = 0.050), and RUNX1 co-mutation (HR 4.445, p = 0.021) were independent prognostic factors (Table 2), but cytogenetics did not reveal significant differences (HR 1.539, p = 0.091, Table 2).
| Risk factors | HR | 95% CI | p |
|---|---|---|---|
| Univariate analysis | |||
| Sex | 0.622 | 0.327–1.184 | 0.148 |
| Age (≤ 65 vs. > 65) | 1.030 | 0.560–1.895 | 0.923 |
|
BM blasts (%) (< 5 vs. 5–10 vs. > 10) |
1.912 | 1.309–2.793 | < 0.001 |
|
RS (%) (< 5 vs. 5–15 vs. > 15) |
1.058 | 0.687–1.628 | 0.799 |
|
Number of mutant genes (1 vs. 2 vs. ≥ 3) |
1.135 | 0.739–1.742 | 0.563 |
| Mutation site | |||
| E622 | 0.534 | 0.128–2.228 | 0.390 |
| R625 | 1.348 | 0.410–4.429 | 0.623 |
| H662 | 0.258 | 0.035–1.881 | 0.181 |
| K700E | 0.967 | 0.522–1.791 | 0.916 |
| K666 | 2.037 | 1.072–3.872 | 0.030 |
| Co-mutation | |||
| TET2 (SF3B1 vs. SF3B1 + TET2) | 1.039 | 0.493–2.189 | 0.920 |
| ASXL1 (SF3B1 vs. SF3B1 + ASXL1) | 1.049 | 0.482–2.282 | 0.904 |
| TP53 (SF3B1 vs. SF3B1 + TP53) | 1.294 | 0.573–2.924 | 0.535 |
|
DNMT3A (SF3B1 vs. SF3B1 + DNMT3A) |
1.143 | 0.502–2.601 | 0.750 |
| RUNX1 (SF3B1 vs. SF3B1 + RUNX1) | 3.106 | 1.087–8.876 | 0.034 |
| STAG2 (SF3B1 vs. SF3B1 + STAG2) | 3.262 | 1.147–9.277 | 0.027 |
| EZH2 (SF3B1 vs. SF3B1 + EZH2) | 0.047 | 0.000–115.304 | 0.443 |
|
Cytogenetics Normal vs. 1 or 2 vs. ≥ 3 |
1.627 | 1.024–2.583 | 0.039 |
| Multivariate analysis | |||
|
BM blasts (%) (< 5 vs. 5–10 vs. > 10) |
1.661 | 1.093–2.524 | 0.017 |
| Mutation site | |||
| K666 | 2.094 | 1.001–4.381 | 0.050 |
| Co-mutation | |||
| RUNX1 (SF3B1 vs. SF3B1 + RUNX1) | 4.445 | 1.254–15.756 | 0.021 |
| STAG2 (SF3B1 vs. SF3B1 + STAG2) | 2.479 | 0.818–7.519 | 0.109 |
|
Cytogenetics Normal vs. 1 or 2 vs. ≥ 3 |
1.539 | 0.943–2.535 | 0.091 |
- Note: Bold part indicates meaningful statistical results.
- Abbreviations: BM, bone marrow; CI, confidence interval; HR, Hazard ratio; RS, ringed sideroblasts.
4 Discussion
The International Working Group for the Prognosis of MDS has proposed SF3B1mut MDS as a distinct disease subtype [1]. In the 5th edition of the classification of hematolymphoid tumors (WHO-HAEM5), MDS with low blasts and SF3B1 mutation was defined as a new subtype [6]. SF3B1mut patients have many similar characteristics, but specific subtypes may show distinct clinical features and prognosis. With further study about the mechanisms of MDS, besides cellular and molecular genetics, immunological therapy also provides a new possibility for SF3B1mut MDS. Therefore, the identification of different mutant subtypes is critical for risk stratification and therapeutic decision-making. In addition, the studies of MDS with SF3B1 mutations should be individualized and comprehensive. To better understand the clinical characteristics and prognosis of SF3B1mut MDS, our research primarily focused on mutant subtypes.
Multiple researches have shown that SF3B1mut patients usually have a lower probability of conversion to AML and are associated with a positive prognosis. The most common clinical feature is anemia. In our cohort, only five patients with the SF3B1 mutation showed no anemia. SF3B1 mutations predominantly involved K700 and, to a lesser extent, K666, H662, and E622 [2]. K700E was the most frequent mutation spot in 47.66% of cases in our cohort, similar to what was noted in previous studies. K666 and R625 were observed in 24.30% and 9.35%, respectively. Two less frequent mutation subtypes accounted for 5.61% of H662 and 4.67% of E622. Kanagal-Shamanna et al. discovered that only patients with the K700E mutation had a good prognosis [11]. A previous study observed that the E622 hotspot was enriched early in the disease and presumed that the E622 hotspot may contribute to indolent MDS [12]. In our study, we further analyzed the clinical features of patients with the E622, R625, and H662 mutations. We did not observe complex karyotypes and AML transformation in patients with the E622 mutation. SF3B1mut patients with the E622 and H622 mutations had higher hemoglobin and a lower percentage of BM blasts. However, when compared to patients with other subtype mutations, the above result did not reach statistical significance due to insufficient samples. In our cohort, 67.3% of the patients had VAF higher than 30%. SF3B1E622 and SF3B1H622 patients showed lower VAF compared to SF3B1non-E622 and SF3B1non-H622 patients, and VAF was significantly lower in SF3B1H662 patients. Further analysis showed that the median OS was longer in patients with E622 and H662 mutations, which was significantly longer than in patients with R625 and K666 mutations. Although the independent positive prognostic value of the E622 and H662 hotspots did not reach statistical significance, the E622 and H662 mutations may be associated with a positive prognosis, which may need to be confirmed by a larger cohort. Patients with the R625 mutation were less likely to show thrombocytopenia, but we did not observe distinct effects on prognosis. Similar to previous findings [12], we observed that SF3B1K666 patients more often showed thrombocytopenia and higher IPSS-R scores. Patients with the K666 mutation were more likely to develop AML. Although most of the patients in this cohort had the normal karyotype, further analysis of the clinical characteristics in this subgroup with specific mutation sites showed no significant differences. Interestingly, the lower percentage of complex karyotype did not change the poor prognosis in MDS with the K666 mutation. In our cohort, the SF3B1 mutation involving K666 independently predicted overall survival in MDS. Interestingly, we observed two patients with double mutation spots. One patient with K700E and E622 double mutation spots, diagnosed with MDS-RS and classified as an intermediate-risk group, showed severe anemia and a poor chromosome karyotype. Another patient with K700E and R939H mutations, diagnosed with MDS-RS and classified as a low-risk group, showed moderate anemia and a good chromosome karyotype.
Previous study has observed that the SF3B1 mutation may be an initiating genetic event [13]. In most patients, SF3B1 may occur as the first event, whereas it may appear to be secondary to other mutations in a minority of cases [14]. In our cohort, four patients did not have the SF3B1 mutation when they were initially diagnosed with MDS, followed by K700E mutations, and showed different clinical features. One showed severe anemia and progressed to death; the other showed moderate anemia; the other showed severe anemia, thrombocytopenia, and leukopenia; and the last one showed thrombocytopenia and leukopenia. These interesting findings allow us to further explore potential mechanisms of splicing mutation in MDS. Our inability to reach statistical significance could be due to insufficient samples. Therefore, research in larger cohorts is warranted.
The SF3B1 mutation had a significant correlation with the percentage of RS [15]. RS is present in about 80% of MDS with SF3B1 mutations. In addition, MDS with low blasts and SF3B1 (MDS-SF3B1), a distinct disease type, includes over 90% of MDS with the percentage of RS ≥ 5 [1]. In our cohort, 75.0% of patients with the SF3B1 mutation had a RS percentage higher than 5%. However, the exact mechanism of the formation of RS in SF3B1mut is complex and unclear. Previous studies have found the SF3B1 mutation may be associated with abnormal gene splicing related to iron metabolism [4, 5, 16]. The disorder of gene expression involved in iron metabolism, such as ABCB7, ALAS2, GATA-1, and MAP3K7 genes, may be associated with the formation of RS. However, the prognostic value of RS in MDS is limited [15]. In our cohort, the percentage of RS was significantly lower in SF3B1mut patients with the K666 mutation compared to those with non-K666 mutations, while significantly higher in SF3B1mut patients with the K700E mutation compared to those with non-K700E mutations. We speculate that mutational subtypes in SF3B1 may cause different gene splicing in iron metabolism, which may lead to changes in the percentage of RS. The STAG2 mutation, as the most frequently mutated cohesin mutation in myeloid malignancies, is essential for chromatin structure and replication [17]. The SF3B1 mutation and STAG2 tend not to occur simultaneously [13]. In our cohort, only 4.7% of SF3B1mut patients had STAG2 co-mutation. Interestingly, we observed a significantly lower percentage of RS in these patients, and three patients had no percentage of RS, which may need to be further studied by a larger cohort due to the insufficient cases with SF3B1 and STAG2 co-mutation.
An excess of 1000 ng/mL of serum ferritin signifies iron overload [18, 19], which may inhibit erythropoiesis by preventing the proliferation of erythrocyte progenitor cells [20, 21]. In addition, when serum erythropoietin exceeds 500 mIU/mL, the reactivity of the erythropoiesis-stimulating drugs (ESA) may decrease [22-24], and the high serum erythropoietin level is associated with lower hemoglobin. In our cohort, only 30.8% of SF3B1mut patients had a serum ferritin level above 1000 ng/mL, whereas 47.7% of patients had a serum erythropoietin level above 500 mIU/mL. We also compared the differences in serum ferritin and serum erythropoietin in SF3B1mut patients with distinct mutation spots. However, we observed no significant difference, which could be due to the limited number of cases and the heterogeneity of our cohort.
SF3B1mut patients with complex mutational status (at least two additional mutations) are associated with a poor prognosis [1, 25]. More than 40% of patients with MDS have at least two mutations, and the co-occurrence of particular genes may change the effect on the prognosis of the SF3B1 mutation. IPSS-R considers hemoglobin, absolute neutrophil count, platelet count, percentage of BM blasts, and cytogenetics, but gene mutations are not used in the risk stratification of patients with MDS. Based on IPSS-R, IPSS-M includes somatic mutations in 31 genes to provide a risk score. In addition, in previous studies, certain genes (TP53, EZH2, ETV6, RUNX1, ASXL1, and BOCR) were associated with an unfavorable prognosis in MDS [26, 27]. On the basis of the above research, Bernard E et al. [7] further divided SF3B1mut MDS into three independent groups: SF3B15q, SF3B1α, and SF3B1β, and found the favorable outcomes were only confined to the SF3B1α group without BCOR mutation, BCORL1 mutation, RUNX1 mutation, NRAS mutation, STAG2 mutation, SRSF2 mutation, and del (5q). Our study revealed that only 17 patients (15.9%) carried SF3B1 as the only mutation in our study. The TET2, ASXL1, TP53, and DNMT3A genes were the most frequently co-occurring mutations with the SF3B1 mutation in our cohort. Therefore, a comprehensive analysis of molecular genetics is important to get a better understanding of the influence of concurrent mutations on prognosis in MDS with the SF3B1 mutation.
In our study, we analyzed the differences in the number of mutations and the most frequent co-occurring mutated genes in SF3B1mut patients with distinct hotspots. We observed that SF3B1mut patients with the E622 mutation were connected to a small number of mutant genes, further supporting the favorable prognosis of the E622 hotspot. It is common knowledge that double-splicing gene mutations are mutually exclusive. In line with the above, we only observed three patients with double-splicing gene mutations. According to the previous study, TET2, DNMT3A, and ASXL1 mutations showed no significant effect on the survival of SF3B1mut MDS patients [7]. In line with the above results, Song et al. [28] found that the co-occurrence of SF3B1 with TET2 had a similar prognosis compared to the single SF3B1 mutation, suggesting the effect of the TET2 mutation on the prognosis of SF3B1mut MDS was minimal. Song et al. [29] found that the positive prognosis of the SF3B1 mutation was not affected by the presence of the DNMT3A mutation. In addition to the above results, some researchers have demonstrated the negative prognosis of the co-occurrence of SF3B1 with ASXL1 [30]. In our study, patients with SF3B1/TET2, SF3B1/DNMT3A, and SF3B1/ASXL1 co-mutations showed limited effects on patients with the SF3B1 mutation. TP53 mutations are associated with complex karyotypes, transformation to AML, and adverse outcomes [31], which especially applies to MDS with multiple TP53 hits, since monoallelic patients showed no difference from TP53 wild-type patients in outcomes in the analysis of the TP53 allelic state [32]. However, little is known about the impact of TP53 concurrent mutations on the prognosis of SF3B1mut MDS. In our cohort, we did not find a significantly adverse effect of the TP53 mutation on clinical features and prognosis in SF3B1mut patients. Further analysis showed most of the mutations in TP53 were mono-hit in patients with the SF3B1 mutation. Malcovati et al. [1] proposed the diagnostic criteria for MDS with mutated SF3B1, and mutations in RUNX1 and EZH2 were the exclusion criteria for the proposed entity. The RUNX1 mutation was associated with worse OS and a higher AML transformation rate [13, 33], while the co-occurrence of SF3B1 with EZH2 may be associated with more severe anemia and transfusion dependency [34]. In line with the previous studies, the RUNX1 co-mutation was an independent adverse prognostic factor in our cohort. However, the EZH2 co-mutation showed no discernible effects on OS. Interestingly, we did not observe RUNX1 and EZH2 mutations in SF3B1mut MDS with the E622 hotspot, further confirming the favorable prognosis of the E622 mutation. Previous studies observed no significantly favorable clinical outcome in SF3B1mut patients with the STAG2 mutation [7]. In line with the above results, we found patients with SF3B1/STAG2 co-mutations were associated with a shorter OS.
The insufficient cases of concurrent mutations and the heterogeneity of molecular genetics in SF3B1mut patients in our study mean that these findings need to be confirmed by analyzing a larger number of cases. Therefore, comprehensive research in molecular genetics is necessary to get a more accurate prognosis for SF3B1mut patients with specific subtypes.
The pathogenesis of MDS involves various factors, and it is not yet fully elucidated. With the exception of molecular genetics and cytogenetics, cellular immune disorders have been confirmed to play a role in the pathogenesis of MDS, although the exact mechanism is unknown. Certain mutations in MDS, especially splicing mutations, contribute to immune disorders. According to previous findings, the activation of inflammatory immune signaling has been observed in SF3B1mut MDS, leading to the corresponding treatment [35]. T cells play a crucial part in the immune response, and changes in the number and function of T cells may lead to immune dysfunction and the expansion of malignant clones [36, 37]. In patients with MDS, different subtypes and stages may have different T cell polarization states. The T cell immune system is associated with the pathogenesis, maintenance, and progression of MDS. Different T cell subsets perform different functions. CD4+ T cells are divided into Th1 and Th2 cells, and the differentiation of Th1/Th2 cells is balanced. Th1 cells mainly mediate cellular immunity and resistance to intracellular pathogens, while Th2 cells mainly mediate humoral immunity and resistance to external pathogens. CD8+ T cells are divided into Tc1 and Tc2 cells, which are crucial to the immunologic defense and surveillance of antitumor immunity. NK cells have intrinsic antitumor activity. Previous studies have observed a decrease in NK cell percentage and impaired function of NK cells in patients with MDS, particularly in high-risk MDS [38]. In addition, the decrease in CD8+ T cell percentage is associated with an increased risk of progressing to AML and decreased overall survival [39].
However, the immune characteristics of mutational subtypes in SF3B1mut MDS have not been analyzed in other cohorts. To understand more information about the immune features of distinct subtypes, we analyzed the differences in T cell and NK cell percentages in the BM. Interestingly, we found SF3B1mut patients with distinct mutation sites showed differences in the polarization of T cells and NK cell percentages. In our study, patients with the K666 mutation showed significantly lower NK cell percentage and the Th1/Th2 ratio in the BM compared to those with non-K666 mutations. Since the polarization of T cells and NK cell percentage are related to antitumor immunity, the decrease in Th1/Th2 ratio and NK cell percentage in the BM in SF3B1mut patients with the K666 mutation may, to some extent, be associated with the poor prognosis in this cohort. These findings indicate that SF3B1mut patients with specific mutational status may have unique immune features, which have not been fully confirmed in other cohorts. Based on the activation of immune signaling in the SF3B1 mutation, we speculate that mutational subtypes in SF3B1 may cause unique abnormalities in gene splicing, which may result in changes in signaling pathways and proteins, which may influence the production and apoptosis of immune cells by interactions of molecules on the cell surface. A better understanding of how different immune cells and MDS cells interact during disease evolution and how immune cells distribute in different subtypes of MDS is helpful in developing more effective therapy. Therefore, the changes in the function of T cells and NK cells may need to be further explored to improve the prognosis of SF3B1mut MDS with unique mutation subtypes.
In summary, SF3B1mut patients with specific mutation spots and concomitant gene mutations showed distinct clinical features and prognosis. Therefore, a comprehensive study of molecular genetics, cytogenetics, and immunity contributes to a better understanding of the pathogenesis, risk stratification, and individualized therapy, which is of great significance to improving the prognosis of patients with SF3B1 mutations.
Author Contributions
Qi Liu: data curation (equal), formal analysis (lead), writing – original draft (lead). Fanhuan Xu: data curation (equal). Juan Guo: data curation (equal). Feng Xu: data curation (equal). Xinhui Huang: data curation (equal). Jianan Chen: data curation (equal). Jiacheng Jin: data curation (equal). Liyu Zhou: data curation (equal). Qi He: data curation (equal). Dong Wu: data curation (equal). Luxi Song: data curation (equal). Zheng Zhang: data curation (equal). Cha Guo: data curation (equal). Jiying Su: data curation (equal). Yumei Zhang: data curation (equal). Meng Yan: data curation (equal). Chunkang Chang: data curation (equal). Xiao Li: data curation (equal). Lingyun Wu: conceptualization (lead), data curation (lead), funding acquisition (lead), project administration (lead), writing – review and editing (lead).
Acknowledgments
This research was funded by the General Program of Shanghai Science and Technology Commission (22ZR1447700).
Ethics Statement
The study was approved by the Ethics Committee of Shanghai Sixth People's Hospital Affiliated to Shanghai Jiao Tong University School of Medicine. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional ethics board of Shanghai Sixth People's Hospital Affiliated to Shanghai Jiao Tong University School of Medicine and were in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent
Informed consent was obtained from all individual participants included in the study.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.