Comprehensive analysis of a new prognosis signature based on histone deacetylases in clear cell renal cell carcinoma
Fajuan Cheng and Bin Zheng are contributed equally to this work.
Funding information
No specific funding was received.
Abstract
Histone deacetylases (HDAC) family is vital for tumorigenesis and tumor progression. However, the exact role of the HDAC family in clear cell renal cell carcinoma (ccRCC) remains unclear. Based on The Cancer Genome Atlas (TCGA), International Cancer Genome Consortium (ICGC), and The Human Protein Atlas (HPA) database, we investigated and validated the expression profile, clinical significance and prognostic value of HDAC family members in ccRCC. Moreover, we further explored the correlation between HDACs and tumor microenvironment, tumor stemness, drug activity and immune subtype. The HDAC8, HDAC10, and HDAC11 manifested potential clinical value for prognosis, and the correlation analyses reveals underlying molecular mechanisms, which deserve further investigation for ccRCC. This Integrated bioinformatics analysis, based on transcriptomics and proteomics, implied that HDAC8, HDAC10, and HDAC11 may serve as potential molecular biomarkers and therapeutic targets for ccRCC, but some underlying molecular mechanisms still need to be elucidated.
1 INTRODUCTION
Kidney cancer is a common urinary carcinoma, with appropriately 73,820 patients newly diagnosed and 14,770 patients die of it in the United States.1 As the most common histological subtype of renal cell carcinoma (RCC), clear cell carcinoma (ccRCC) always leads to a poor survival rate. It is estimated that nearly 25%–30% ccRCC patients have metastasized by the initial diagnosis time.2, 3
With the understanding of human genetics, medical oncology had revolutionized. Epigenetics, a heritable alteration in gene expression, make genetic material package effectively.4 As one of three interlinked epigenetic modifications, histone covalent modification, especially histone acetylation, plays an indispensable role in the expression status of promoters.5 Recently, numbers of epigenetic mechanism of oncogenesis have been analyzed to its finer detail. In RCC, histone modification is strongly associated with the increased risk of poor prognosis. For example, reduction in H3Ac, H4Ac, H3K18Ac, and H3K27me are related to poor clinical outcomes in RCC patients, such as recurrence, metastasis, worse cancer-specific survival, and progression-free survival.6-8
Histone deacetylases (HDACs), consisting of 18 conserved genes, are divided into four classes: class Ⅰ (HDAC1, HDAC2, HDAC3, HDAC8), class Ⅱ (HDAC4, HDAC5, HDAC6, HDAC7, HDAC9, HDAC10), class Ⅲ (SIRT1 – SIRT7), and class Ⅳ (HDAC11).9 Previous evidence suggested that HDACs in class Ⅰ overexpressed in ccRCC and HDACs in class Ⅱ regulated ccRCC biological functions.10, 11 However, research concerning about HDACs in ccRCC biology and prognosis is still lacking. Because HDAC inhibitors mainly target class Ⅰ, Ⅱ, and Ⅳ, which are also known as classical HDACs,12 we evaluated these 11 genes in this study. We hope that this study could contribute to the understanding of novel molecular therapeutic targets for ccRCC patients.
2 MATERIALS AND METHODS
2.1 Data collection and analysis
The expression data and clinical information of ccRCC and 33 cancers were downloaded directly from the ICGC (https://dcc.icgc.org/) and UCSC Xena database (https://xena.ucsc.edu/). Stemness score data and immune subtype were also downloaded from the UCSC Xena database.40 All gene expression data were normalized by “limma” package.
We assessed the differentially expressed genes (DEGs) in paired tumor as well as non-tumor tissues by the “limma” R package and visualized by “pheatmap” and “vioplot” R package. Correlation between HDACs and stemness was performed by “corrplot” package. Correlation between HDACs and immune subtype was visualized by “ggplot2” package. The protein–protein interaction (PPI) network was performed for all HDACs via the STRING database (http://string-db.org/).
LASSO regression analysis, univariate, and multivariate Cox regression analyses were separately conducted by "glmnet" and “survival” package.41 Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses were performed through "clusterProfiler" package. Single-sample gene set enrichment analysis (ssGSEA) was performed by the "gsva" package.
2.2 Construction and validation of the prognostic signature
The prognostic-related genes were selected after performing univariate Cox analysis with p < 0.05. To minimize the risk of overfitting and choose optimal genes, LASSO regression analysis was conducted. Then, the median value of the risk score was calculated by this formula: Risk score = 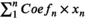 (Coefn is the coefficient and xn is the expression level of each genes). Based on median risk scores, ccRCC samples were stratified into high- and low-risk sets. Afterward, the Kaplan–Meier (K-M) curve, PCA analysis, and distribution of risk scores were executed to assess the accuracy of the established prognostic model. We verified our model via the HPA database (https://www.proteinatlas.org/).
(Coefn is the coefficient and xn is the expression level of each genes). Based on median risk scores, ccRCC samples were stratified into high- and low-risk sets. Afterward, the Kaplan–Meier (K-M) curve, PCA analysis, and distribution of risk scores were executed to assess the accuracy of the established prognostic model. We verified our model via the HPA database (https://www.proteinatlas.org/).
2.3 GSCAlite database, CellMiner database, Oncomine database, and UALCAN analysis
We used GSCALite database (http://bioinfo.life.hust.edu.cn/web/GSCALite/) to explore SNV and CNV of HDACs in pan-cancer and the degree of Cancer pathway activity.42 The UALCAN online tool (http://ualcan.path.uab.edu/) was utilized to analyze protein expression in ccRCC.43 CellMiner database (https://discover.nci.nih.gov/cellminer/home.do) was utilized to integrate HDACs and pharmacological data.44 Oncomine dataset (www.oncomine.org) was employed to compare the expression profile of HDACs in pan-cancers.45
2.4 ESTIMATE algorithm
We used ESTIMATE (Estimation of Stromal and Immune cells in Malignant Tumor tissues using Expression data) algorithm to measure stromal scores and immune scores to predict the infiltration of stromal and immune cells among 33 tumors (https://portal.gdc.cancer.gov/).45
2.5 Statistical analysis
Mann–Whitney test was utilized to measure gene expression level. We eliminate samples that clinical information is lost or unknown. The K–M curve with log-rank test was adopted in survival analysis. Statistical analysis was performed with R packages (R version 4.0.1). A two-tailed p < 0.05 was considered significant.
2.6 Nomenclature
aDC: Activated dendritic cell; APC: Antigen-presenting cell; CCR: Cytokine–cytokine receptor; CI: Confidence interval; EMT: Epithelial-Mesenchymal Transition; FDR: False discovery rate; HLA: Human leukocyte antigen; HR: Hazard ratio; iDC: Immature dendritic cell; LASSO: Least absolute shrinkage and selection operator; MHC: Major histocompatibility complex; PCA: Principal component analysis; Tfh: T follicular helper cell; TIL: Tumor-Infiltrating Lymphocyte.
3 RESULTS
3.1 The differential expression pattern of HDACs in pan-cancers
We investigated the mRNA expression by using Oncomine database. As can be seen from Figure 1A, several genes (HDAC1, HDAC2, HDAC3, HDAC7, HDAC8, HDAC9) express highly in most cancers, such as brain and CNS cancer, kidney cancer, breast cancer, and leukemia, which presents its potential role in pan-cancer. Besides, we also analyzed the expression level of HDACs proteins by UALCAN database in breast cancer, colon cancer, ovarian cancer, clear call renal cancer, uterine corpus endometrial carcinoma, and lung adenocarcinoma. The results of boxplot also show that there is a significantly different expression level in HDAC family, which confirmed the above results (Figure S1–S6). We also verified these results by downloading mRNA expression data from TCGA database. As shown in Figures 1B and S7, these results are consistent with results from the Oncomine database.
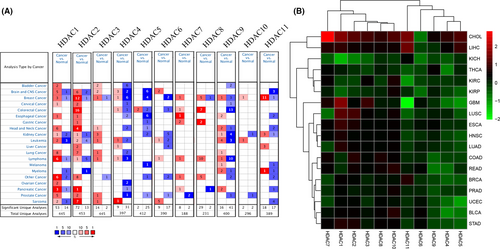
3.2 Genetic alterations of HDACs in pan-cancers
Due to gene mutation is instrumental in tumorigenesis, we illustrated the single nucleotide variations (SNV) and copy number variations (CNV) of HDACs in pan-cancers by utilizing the GSCAlite database. First, as shown in Figure S8A, HDAC9 and HDAC7 have heterozygous amplifications in most of cancers, and HDAC8, HDAC6, HDAC4, HDAC1, HDAC2, HDAC10, and HDAC11 have apparently heterozygous mutations in the majority of cancers as well. The results from SNV analysis indicate that HDAC9, HDAC4, HDAC6, HDAC5, HDAC7, HDAC3, HDAC2, HDAC11, HDAC10, and HDAC8 are the top 10 mutated genes, with mutation rates from 7% to 32% (Figure S8B,C). Besides, the missense mutation occupies the most part in numerous types of mutations and appears more frequently in uterine endometrial carcinoma (Figure S8B,C).
3.3 Prognostic significance of HDACs in pan-cancers
Considering the above results of HDACs in pan-cancers and limited prognostic data of HDAC family, we then assessed the prognostic value of HDACs in pan-cancers through utilizing overall survival (OS) data from the TCGA. This revealed that in most of cancers, low expression of HDACs could lead to a better survival condition (Figure S9). However, in certain cancers, such as adrenocortical carcinoma, bladder urothelial, cervical squamous cell carcinoma, and cholangiocarcinoma, several HDAC family members act as favor prognosis biomarkers. For example, in RCC (including chromophobe carcinoma, ccRCC, and papillary cell carcinoma), low expression of HDAC1, HDAC2, HDAC3, HDAC8, HDAC10 and high expression of HDAC5, HDAC7, HDAC11 have longer survival time.
The existing evidence suggested that various HDAC inhibitors, including LBH589 and OBP-801, could promote RCC cell apoptosis and ameliorate the outcomes of RCC patients.13, 14 Given the potential clinical value of the HDAC family in ccRCC, we next conducted a comprehensive analysis of HDACs in ccRCC.
3.4 HDAC family expression in ccRCC
The expression heatmap and violin plot indicate the mRNA expression level of HDAC family (Figure 2A,B). Most of genes (9/11, 81.8%) show significantly difference expression in ccRCC samples, and among these genes, HDAC3, HDAC7, and HDAC10 have higher expression level, compared with normal samples. Moreover, the expression of HDAC1, HDAC2, HDAC4, HDAC6, HDAC8, and HDAC11 present a significant decrease in ccRCC samples. For the last two genes, HDAC5 and HDAC9, the apparently statistic difference was not observed between normal samples and ccRCC samples. The above results suggest that HDAC family may have a huge influence on ccRCC.
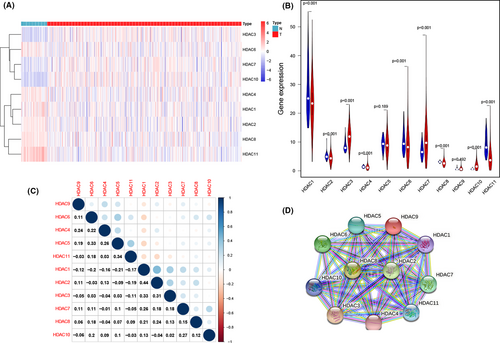
We also analyzed the interactive relationship among all HDACs genes. The results suggested that most of these genes had a positive correlation and the most significant paired genes are HDAC2 and HDAC1 (r = 0.44) (Figure 2C). The PPI network shows that HDAC2 and HDAC8 are hub genes (Figure 2D).
3.5 Construction of the HDAC-based risk signature
Due to limited study investigated the potential prognostic value of HDACs in ccRCC. we applied the univariate Cox regression analysis with p < 0.05 to select the prognostic-related genes, and the results reveal that the expression of HDAC7, HDAC8, and HDAC10 are positively correlated with survival rates and the expression of HDAC5, HDAC11 are negatively correlated with survival rates in ccRCC patients (Figure 3A). After putting all these genes into LASSO regression analysis, the final signature was built by HDAC8, HDAC10 and HDAC11. All patients were divided into low and high sets based on median risk scores. Next, we employed K–M analysis with a 95% confidence interval. The results clearly indicate that patients in low-risk set have a better survival time (p < 0.05) (Figure 3B). Then, the ROC curve demonstrates that the risk signature has an acceptable efficiency (AUC = 0.686) and the PCA analysis based on the risk signature successfully distinguish two risk set (Figure 3C,D). The risk scores of three genes and corresponding expression profiles are shown in Figure 3E,F. Overall, the results demonstrate that the three-gene risk signature could effectively filter out high-risk ccRCC patients with poor clinical outcomes. From Figure 3G,H, we can observe that patients’ age (HR = 1.513, 95% CI [1.108–2.065], p = 0.009), tumor stage (HR = 3.099, 95% CI [2.016–4.765], p < 0.001), and risk score (HR = 2.534, 95% CI [1.902–3.375], p < 0.001) are associated with worse OS.
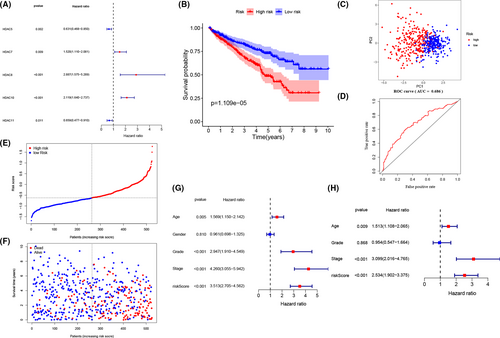
3.6 Validation of the HDAC-based risk signature
To valid the above results, we used 92 ccRCC patients with complete clinical information from ICGC database, which contains. We observed that the risk model demonstrated the same trend when ccRCC patients from ICGC database were separated into high- and low set at median risk scores (Figure 4A–E). The results from K–M analysis and the risk scores present that high-risk patients have worse survival condition and the ROC curve presents that risk score has an acceptable predictive ability (AUC = 0.600) (Figure 4A,B). Moreover, we found that in the ICGC validation set, tumor stage (HR = 2.565, 95% CI [1.066–6.171], p = 0.036) and risk score (HR = 1.707, 95% CI [1.093–2.665], p = 0.019) are correlated with worse OS (Figure 4F,G). Finally, we validated the immunohistochemistry pattern by utilizing the HPA database.
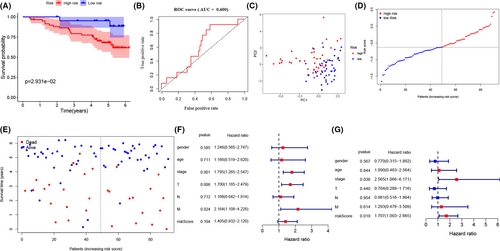
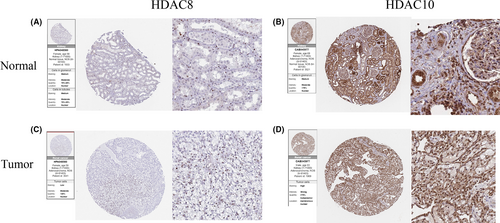
We noticed that normal kidney tissue staining of HDAC8 exhibits medium staining in nuclear of tubules cells (Figure 5A). Instead, the weak staining was located in the nuclear of tumor cells. (Figure 5C). For HDAC10, moderate staining patterns were positive on the cell membrane and nuclear of tubules cells in normal kidney tissues (Figure 5B), but as for renal cancer samples, the high staining was observed on these cellular structures (Figure 5D). These results, not only corroborate the above findings but also assist clinicians to predict the clinical prognosis of patients.
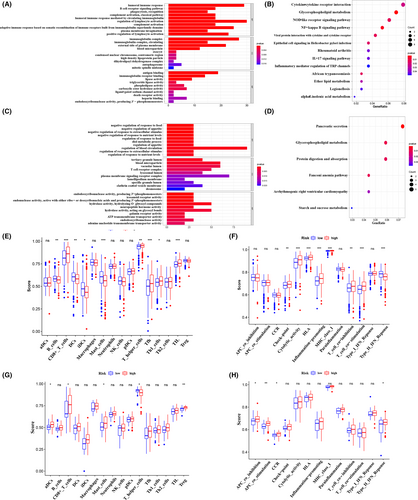
3.7 Biological functions analysis
To interrogate the biological functions and pathways behind HDACs in TCGA and ICGC cohorts, we performed the GO and KEGG analysis. In TCGA cohort, GO terms are enriched in humoral immune response, positive regulation of lymphocyte activation, regulation of lymphocyte activation, complement activation, classical pathway, B-cell receptor signaling pathway, and so on (Figure 6A). Moreover, the associated KEGG pathways are enriched in glycerophospholipid metabolism, cytokine−cytokine receptor interaction, NF−kappa B signaling pathway, IL−17 signaling pathway, and so on (Figure 6B). In ICGC ccRCC cohort, GO terms are enriched in lysosomal lumen, T-cell receptor complex, hydrolase activity, plasma membrane signaling receptor complex, ATP transmembrane transporter activity, and so on (Figure 6C). For KEGG analysis, the associated networks are enriched in glycerophospholipid metabolism, protein digestion and absorption, starch and sucrose metabolism, and so on (Figure 6D).
Then, the GSCAlite database was utilized to figure out the underlying role of HDACs in classical pathways, which turned out that HDACs may activate or inhibit several oncogenic pathways. For instance, HDAC11 may activate PI3K/AKT pathway and inhibit apoptosis, cell cycle, and EMT pathways (Figure S10A,B).
Considering the obvious enrichment in various immune-related processes. We utilized the ssGSEA to calculate enrichment scores of immune cells as well as immune functions. As shown in Figure 6E,F, the enrichment scores of certain immune cells, including CD8+ T cell, T helper cell, Th1 cell, Tfh cell, TIL cell, and immune functions such as check-point, cytolytic activity, and HLA are significantly high in high-risk set of TCGA ccRCC patients (p < 0.05). For ICGC cohort, aDCs cell, mast cells, Treg cells, APC co-inhibition, APC co-stimulation have high scores in high-risk group (p < 0.05) (Figure 6G,H).
3.8 Correlation analyses of HDAC family in ccRCC
The immune subtype boxplots (Figure 7A) and clinic correlation boxplots (Figure 7B–M) show the expression within HDACs between immune subtypes and clinicopathological features separately. We noticed that HDAC1 expressed eminently among six immune subtypes, and the expression level in C1 type was obviously higher than other types (p < 0.05) (Figure 7A).
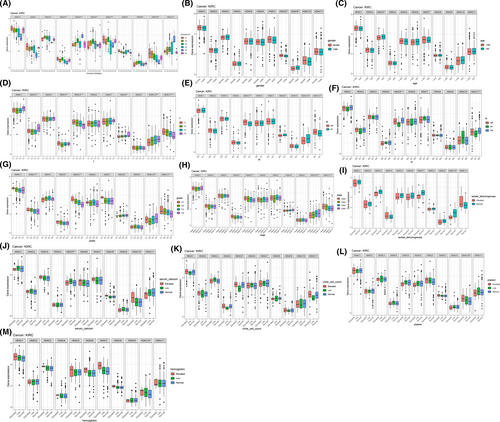
The outcomes of the correlation analysis reveal that the expression of some HDACs is strongly correlated with clinicopathological characteristics. For example, the expression of HDAC3, HDAC9 are significantly associated with patients’ age and gender separately. Specifically, patients older than 65 or male patients have higher expression level. Moreover, the expression of HDAC5 and HDAC11 are positively parallel with tumor stage and grade, T, N, and M stage. Some laboratory markers are also bound up with the expression of HDACs. For instance, the expression of HDAC10 is associated with the level of platelet, lactate dehydrogenase, and hemoglobin (all p < 0.05); the expression level of HDAC5 and HDAC8 are associated with the level of serum calcium (all p < 0.05) and the expression level of HDAC11 is correlated with the count of white blood cell (p < 0.05) (Figure 7B–M).
Next, we scrutinized the relationship between HDACs and tumor microenvironment, as well as tumor stemness (Figure S11A). The consequence indicates that most of HDACs are linked with tumor stemness, stromal cell, and immune cells. For example, the expression level of HDAC2 is negatively related to tumor stemness (data based on RNA expression) (p = 0.035), and the higher the expression level of HDAC2, the more stromal cells (p < 0.001) instead of immune cells in the tumor microenvironment. In addition, given several genes mutations have been described in ccRCC, we also conducted the correlation analysis between HDAC8, HDAC10, HDAC11, and common mutated genes, including VHL, PBRM1, mTOR, and BAP115 (Figure S12,A-I). The result indicates that all HDACs are positively correlated with common mutated genes, except that HDAC10 has no correlation with PBRM1 (Figure S12E).
Finally, the correlation analysis of all HDACs and drug activity was analyzed using data from the CellMiner database (Table S1; Figure S11B). The top 16 relevant correlations between HDACs and drug activity are shown in Figure S11B. We found that the activity of some commonly used drugs is negatively related to the expression level of HDAC11, including oxaliplatin, carmustine, ifosfamide, lmexon, lomustine, and BN-2629. In addition, the activity of some common drugs has a positive relationship between HDACs expression as well. By way of illustration, the sensitivity of temsirolimus, a specific inhibitor of mTOR and HDAC inhibitor vorinostat are positively correlated with HDAC10 expression (r = 0.315, p = 0.014).
4 DISCUSSION
With the huge revolution and decreased cost of gene sequencing, a vast amount of genetic information is easily accessible to researchers.16 However, most of the precision treatments targeting at gene alterations are seldom available. At the same time, under the huge pressure of high cost, only a limited percentage of patients could benefit from precise treatments.17, 18 Nowadays, faced with a limited understanding of the biological relationship between tumor genotype and phenotype, some pivotal molecular signatures need to be explored.
It is reported that histone modification, such as H3Ac, H4Ac, H3K18Ac, and H3K27me3, plays a pivotal role in tumorigenesis and progression.8, 19 Histone acetylation, as a common type of histone modifications, involves many enzymes that could add or remove acetylation markers,6 which implies that histone acetylation may serve as a potential target for tumor therapy. Recent literature has demonstrated that inhibiting histone deacetylase (especially HDAC2) could reverse drug resistance to angiogenesis inhibitors in RCC patients.20 HDAC8 is a multifaceted target for therapeutic interventions in colon, lung, and hepatocellular carcinoma cervical cancers as well, which regulates proliferation and apoptosis in cancer cells.21, 22 HDAC10 were also found to be prognostic markers for gastric cancer and colon cancer.23, 24 Reports in PNAS and Autophagy illustrate that HDAC10 could promote autophagy-mediated survival in neuroblastoma and improve treatment response of advanced neuroblastomas.25, 26 In lung cancer, HDAC10 is positively associated with the expression level of PD-L1, which acts as an independent prognostic factor27 and regulates stem-like lung adenocarcinoma cell.28 Fan W et al29 found that HDAC10 expression was suppressed in ccRCC and also involved in the development and metastasis of ccRCC. Moreover, prior research noted that HDAC11 was a novel prognostic marker, affecting apoptosis and maintaining the metabolism and viability of cancer cells in prostate cancer, pancreatic cancer, ovarian and breast cancer.30, 31 As few researchers focus on other members of HDAC family, their roles in ccRCC are still far from being known and require further investigation.
We explored here the expression profile of HDAC family via Oncomine database, which indicated that HDAC1, HDAC2, HDAC3, HDAC7, HDAC8, and HDAC9 expressed highly in pan-cancer at transcriptional level. Then, the same trend was observed by using TCGA RNA-seq data. We also utilized the CTPAC protein expression data from UALCAN online tools to analyze HDACs expression profile on protein level, and the results were the same as the RNA transcriptional data as well. Considering the key role of tumor mutations in regulating anticancer immunity,32 we analyzed the SNV and CNV of HDACs in pan-cancers by utilizing the GSCAlite database. The results turned out that most of HDACs mutated at pan-cancer, and missense mutation occupied the most part in all types of mutations. Then, we exploit overall survival and identified that low expression level of HDAC1, HDAC2, HDAC3, HDAC8, HDAC10, and high expression level of HDAC5, HDAC7, and HDAC11 had better survival in RCC. To deeply explore the prognostic value of HDAC family in ccRCC, we filtered out HDAC8, HDAC10, and HDAC11 and constructed a risk signature after univariate Cox analysis and LASSO regression analysis by data from the TCGA database. The results from K–M curves, risk score profiles, and multivariate Cox analysis all illustrated a favorable role in risk prediction. In our validation cohort, including patients in the ICGC database and HPA database, we still observed a significantly differential survival trend for the risk model, which suggested the accurate efficiency of the risk model consisting of HDAC8, HDAC10, and HDAC11. The underlying mechanisms were interpreted by GO analysis, KEGG analysis, ssGSEA analysis, and correlation analyses between HDACs and immune subtypes and tumor stemness. The results presented that immune-associated functions and pathways were enriched in HDACs, and most of HDACs are related to tumor stemness, stromal cell, and immune cells, which is consistent with previous studies.33-35 Besides, further validation from the GSCAlite database demonstrated that HDACs are involved in PI3K/AKT pathway and EMT pathways. Finally, for the assessment of clinical value, we validated the relationship between HDACs and clinicopathological characteristics, as well as drug activity. Our finding indicated that patients’ age and gender, tumor stage, and grade, T, N, and M stage, and laboratory markers (platelet, lactate dehydrogenase, serum calcium, white blood cell count, and hemoglobin) were significantly associated with HDACs expression. Noticeably, the activity of some commonly used drugs (such as oxaliplatin, vorinostat, temsirolimus) is also influenced by HDACs.
Nowadays, with the first approval of vorinostat (a HDAC inhibitor) by FDA, more HDAC inhibitors have been used to treat malignant tumors. In RCC, using vorinostat alone or combining vorinostat and temsirolimus inhibited the proliferation and angiogenesis in vitro and vivo models.36, 37 Moreover, the combination of HDAC inhibitor valproic acid and everolimus may hinder drug resistance caused by long-term everolimus treatment.38 However, the majority of HDAC inhibitors were applied to hematological tumors, and unfortunately the demonstrated effect in solid tumors is not as effective as hematological tumors.39 Therefore, further exploration of HDAC biological functions and rapid development of potent-specific inhibitors is of the essence.
To improve the outcome of ccRCC and the effect of HDAC inhibitor, it is necessary to identify ccRCC patients who could benefit the most from treatments at the first diagnosis. Therefore, accurate and efficient biomarkers are indispensable. Our study purposed to reveal the molecular mechanism as well as clinical value, and the results suggest HDAC8, HDAC10, and HDAC11 could be used to estimate patients’ prognosis and serve as potential therapeutic targets. In future investigations, patients’ data from our center will be collected to valid this signature and further experiments in vivo and vitro will be implemented to confirm the possibility as prognostic biomarkers as well.
ACKNOWLEDGMENT
None.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
B.Z. and W.H. involved in conceptualization; B.Z., F.J.C., Z.S.Y., and G.T.Z. involved in methodology; B.Z. and J.W.W. carried out software preparation; F.J.C., B.Z. also carried out writing of the manuscript; and Z.H.N., W.H. involved in project administration.
ETHICAL APPROVAL STATEMENT
None.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings are available from the TCGA and ICGA databases.