Pancreatic cancer survival trends in the US from 2001 to 2014: a CONCORD-3 study
Abstract
Background
Survival from pancreatic cancer is low worldwide. In the US, the 5-year relative survival has been slightly higher for women, whites and younger patients than for their counterparts, and differences in age and stage at diagnosis [Corrections added Nov 16, 2022, after first online publication: a new affiliation is added to Maja Nikšić] may contribute to this pattern. We aimed to examine trends in survival by race, stage, age and sex for adults (15-99 years) diagnosed with pancreatic cancer in the US.
Methods
This population-based study included 399,427 adults registered with pancreatic cancer in 41 US state cancer registries during 2001-2014, with follow-up to December 31, 2014. We estimated age-specific and age-standardized net survival at 1 and 5 years.
Results
Overall, 12.3% of patients were blacks, and 84.2% were whites. About 9.5% of patients were diagnosed with localized disease, but 50.5% were diagnosed at an advanced stage; slightly more among blacks, mainly among men. No substantial changes were seen over time (2001-2003, 2004-2008, 2009-2014). In general, 1-year net survival was higher in whites than in blacks (26.1% vs. 22.1% during 2001-2003, 35.1% vs. 31.4% during 2009-2014). This difference was particularly evident among patients with localized disease (49.6% in whites vs. 44.6% in blacks during 2001-2003, 60.1% vs. 55.3% during 2009-2014). The survival gap between blacks and whites with localized disease was persistent at 5 years after diagnosis, and it widened over time (from 24.0% vs. 21.3% during 2001-2003 to 39.7% vs. 31.0% during 2009-2014). The survival gap was wider among men than among women.
Conclusions
Gaps in 1- and 5-year survival between blacks and whites were persistent throughout 2001-2014, especially for patients diagnosed with a localized tumor, for which surgery is currently the only treatment modality with the potential for cure.
Abbreviations
-
- CI
-
- confidence interval
-
- ICD-O-3
-
- International Classification of Diseases for Oncology
-
- SEER
-
- Surveillance Epidemiology and End Results
-
- US
-
- United States
1 BACKGROUND
Pancreatic cancer is one of the most lethal cancers worldwide and the third most common cause of death from cancer in the United States (US) [1-4]. Its incidence has been increasing by about 1% each year, and mortality by 0.3% per year [5].
The main reason behind the poor prognosis is likely to be advanced-stage disease at diagnosis due to the vague nature of symptoms and rapid tumor progression, and only about 10% are operable at diagnosis [4]. The only potentially curative treatment, radical surgery, combined with adjuvant chemotherapy, has been associated with a moderate improvement in survival [6].
Survival from pancreatic cancer is lower than for most other cancers. In the CONCORD-3 study [7], the age-standardized 5-year net survival generally ranged between 2.2% and 19.0% worldwide, with little improvement over time. In the US, survival improved slightly, from 7.2% for patients diagnosed during 2000-2004 to 8.9% in 2005-2009 and 11.5% in 2010-2014.
Pancreatic cancer incidence in the US is higher in African-Americans (blacks) than in whites [8]. The evidence on racial disparities in survival is less consistent. Five-year survival was slightly higher in whites than in blacks during 2010-2016, especially among men [9]. Other reports also suggest lower survival among blacks than whites [10-14], but these differences have not been seen in all studies [15-18].
Pancreatic cancer survival in the US is slightly higher among women than among men, in younger than in older patients, and for patients with localized disease than for those with advanced disease [9]. We aimed to examine the distribution and trends in net survival up to 5 years, stratified by race, stage at diagnosis, age and sex, for adults (15-99 years) diagnosed with pancreatic cancer during 2001-2014 in the US, overall and by US state.
2 METHODS
2.1 Data sources
We examined data from the CONCORD-3 study (https://csg.lshtm.ac.uk/concord) for 458,895 adults diagnosed with a primary, invasive, malignant tumor of the pancreas by anatomic site [International Classification of Diseases for Oncology (ICD-O-3 [19]) codes C250-C254 and C257-C259] and behavior (ICD-O-3 code 3).
The third cycle of the CONCORD program updated the global surveillance of trends in cancer survival to include patients diagnosed during 2000-2014 and followed up to 31 December 2014 [7]. Overall, the study included data for over 37.5 million patients diagnosed with one of 18 cancers or groups of malignancies, including pancreatic cancer, worldwide.
Data for CONCORD-3 were provided by 322 population-based cancer registries in 71 countries, 47 of which provided data with 100% coverage of the national population [7]. In the US, 42 state registries provided data for CONCORD-3. Missouri and Washington State were not included in these analyses because Missouri did not provide data on pancreatic cancer, and data from Washington State were only available for patients diagnosed up to December 31, 2008. Maryland later submitted data for this study and was included (Supplementary Table S1). In all, these 41 states provided 85% coverage of the US population in 2014.
2.2 Quality control
Detailed descriptions of data acquisition, ethical approval and data quality control have been published [7]. Briefly, we considered primary, invasive tumors of the pancreas for survival analyses. Tumors registered only from a death certificate or discovered at autopsy were excluded because their duration of survival was unknown. We also excluded records with incomplete data or with an invalid date or sequence of dates, or for patients whose sex or vital status was unknown, or whose age was outside the range of 15-99 years. We also excluded benign tumors (ICD-O-3 behavior code 0), or with behavior coded as uncertain (1), in situ (2), metastatic from another organ (6) or unknown (9). After these exclusions, the records of 423,774 patients (93.7% of those eligible) remained for inclusion in survival analyses (Supplementary Table S1).
2.3 Statistical analysis
Patients were categorized by race (whites, blacks, others) and stage at diagnosis (localized, regional, distant or unknown) using Surveillance Epidemiology and End Results (SEER) Summary Stage 2000 [20].
We also excluded 24,347 patients diagnosed in 2000 (Supplementary Table S1) because the CONCORD-3 protocol only required data on stage at diagnosis for patients diagnosed in 2001 or later; data on stage at diagnosis for 2000 in the US and other countries were considered insufficiently complete.
We analyzed survival for patients diagnosed in three calendar periods: 2001-2003, 2004-2008 and 2009-2014. This choice enabled us to use the cohort approach [21] for patients diagnosed during 2001-2003 and 2004-2008, for all of whom at least 5 years of follow-up data were available by December 31, 2014, and the complete approach [22] for patients diagnosed during 2009-2014, for whom 5 years of follow-up data were not available for all patients. The choice was partly dictated by changes in the data collection methods for the stage at diagnosis. From 2001, most US registries coded stage at diagnosis to SEER Summary 2000 stage directly from the source data [20]. From 1 January 2004, US registries began to derive SEER Summary Stage 2000 from 15 pathological and clinical data items using the Collaborative Staging System [23]. This choice of calendar periods was designed to minimize the impact of these changes in data collection methods on estimates of the trends in survival by stage. Data on stage at diagnosis were available for at least 70% of patients in the 41 states for all three calendar periods.
We estimated net survival with the Pohar Perme estimator [24]. Net survival is the cumulative probability for cancer patients to survive their cancer up to a given time since diagnosis, after controlling for competing risks of death (background mortality). To account for differences in background mortality between states, blacks and whites, men and women, and trends over time, we used life tables of all-cause mortality rates by single year of age, sex, race and single calendar year for each state [25].
We present estimates of net survival at 1 and 5 years after diagnosis, by sex and stage at diagnosis, for all patients and separately for blacks and whites, and for two broad groups of age at diagnosis (15-64 and 65-99 years). We also estimated net survival for five age groups (15-44, 45-54, 55-64, 65-74 and 75-99 years) to present the conventional age-standardized survival estimates for all ages combined, using the International Cancer Survival Standard weights [26].
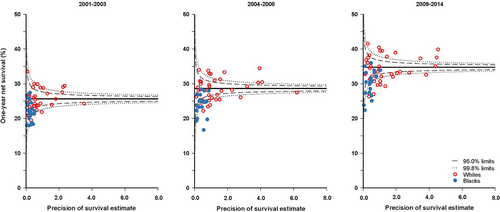
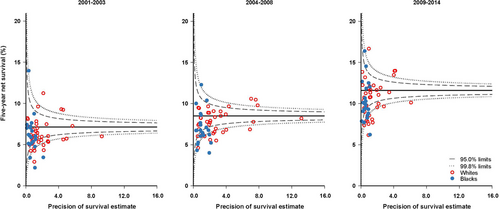
We produced funnel plots of age-standardized net survival for each calendar period, by race and state. These plots show the variability of cancer survival in the US by race and state, showing the extent to which survival estimates vary within the 95% and 99.8% control limits around the pooled estimate for the 41 participating states (the “target” estimate), given the precision of each estimate.
It was not possible to produce robust age-standardized estimates for blacks and whites in every state for all three calendar periods. We did not estimate survival if fewer than 10 patients were available for analysis. If 10-49 patients were available for analysis, we only estimated survival for all ages combined. If 50 or more patients were available, we attempted to estimate survival for each age group. If a single age-specific estimate could not be obtained, we merged the data for adjacent age groups and assigned the combined estimate to both age groups before standardization for age. If two or more age-specific estimates could not be obtained, we present only the unstandardized estimate for all ages combined. We did not merge data between consecutive calendar periods. For these reasons, the funnel plots include age-standardized survival estimates for 1-year and 5-year survival estimates from 40 of the 41 states for whites and 21 states for blacks.
3 RESULTS
3.1 Patients’ characteristics
We included data for 399,427 pancreatic cancer patients in the analyses, of whom 50.4% were men; 84.2% of patients were whites, 12.3% blacks (Table 1), and 3.5% of other ethnic or racial groups, including Asian or Pacific Islander, American Indian/Alaska Native, and other unspecified or unknown races (0.4%, data not shown). Most patients were aged 65 years or older (66.4%), and more than half were diagnosed at distant stage (50.5%). Only 9.5% of patients were diagnosed with a localized cancer. The stage at diagnosis was unknown for 11.1% of patients.
| Population | Variable | All races [No. (%)] | Whites [No. (%)] | Blacks [No. (%)] |
|---|---|---|---|---|
| The whole cohort | Total | 399,427 (100.0) | 336,479 (84.2) | 49,102 (12.3) |
| Sex | ||||
| Men | 201,383 (50.4) | 171,908 (51.1) | 22,654 (46.1) | |
| Women | 198,044 (49.6) | 164,571 (48.9) | 26,448 (53.9) | |
| Age at diagnosis | ||||
| 15-64 years | 134,058 (33.6) | 107,713 (32.0) | 21,162 (43.1) | |
| 65-99 years | 265,369 (66.4) | 228,766 (68.0) | 27,940 (56.9) | |
| Stage at diagnosis | ||||
| Localized | 37,998 (9.5) | 32,034 (9.5) | 4,678 (9.5) | |
| Regional | 115,318 (28.9) | 97,712 (29.0) | 13,450 (27.4) | |
| Distant | 201,631 (50.5) | 168,802 (50.2) | 25,910 (52.8) | |
| Unknown | 44,480 (11.1) | 37,931 (11.3) | 5,064 (10.3) | |
| Men | Total | 201,383 (100.0) | 171,908 (85.4) | 22,654 (11.2) |
| Age at diagnosis | ||||
| 15-64 years | 77,204 (38.3) | 62,917 (36.6) | 11,452 (50.6) | |
| 65-99 years | 124,179 (61.7) | 108,991 (63.4) | 11,202 (49.4) | |
| Stage at diagnosis | ||||
| Localized | 17,519 (8.7) | 15,050 (8.8) | 1,866 (8.2) | |
| Regional | 57,585 (28.6) | 49,674 (28.9) | 5,968 (26.3) | |
| Distant | 106,284 (52.8) | 90,035 (52.4) | 12,662 (55.9) | |
| Unknown | 19,995 (9.9) | 17,149 (10.0) | 2,158 (9.5) | |
| Women | Total | 198,044 (100.0) | 164,571 (83.1) | 26,448 (13.4) |
| Age at diagnosis | ||||
| 15-64 years | 56,854 (28.7) | 44,796 (27.2) | 9,710 (36.7) | |
| 65-99 years | 141,190 (71.3) | 119,775 (72.8) | 16,738 (63.3) | |
| Stage at diagnosis | ||||
| Localized | 20,479 (10.3) | 16,984 (10.3) | 2,812 (10.6) | |
| Regional | 57,733 (29.2) | 48,038 (29.2) | 7,482 (28.3) | |
| Distant | 95,347 (48.1) | 78,767 (47.9) | 13,248 (50.1) | |
| Unknown | 24,485 (12.4) | 20,782 (12.6) | 2,906 (11.0) | |
The proportion of men was higher among whites than among blacks (51.1% vs. 46.1%), as was the proportion of patients aged 65-99 years (68.0% vs. 56.9%) (Table 1). The age profile of black men with pancreatic cancer was younger than that of white men, whereas both white and black women were generally diagnosed at older ages than their male counterparts (Table 1). These differences were relatively stable during all three calendar periods (Supplementary Tables S2-S3).
Diagnosis at a localized stage was uncommon in both whites and blacks (9.5%) and similar in both sexes (Table 1). Black patients were slightly more likely to be diagnosed at a distant stage than white patients (52.8% vs. 50.2%), among both men (55.9% vs. 52.4%) and women (50.1% vs. 47.9%) (Table 1). Differences in the stage distribution between blacks and whites changed very little during 2001-2014 (Supplementary Tables S2-S3). The proportion of records with unknown stage fell from 14.1%-18.8% during 2001-2003 to 7.3%-9.1% during 2009-2014, with similar improvements among men and women, and among blacks and whites (Supplementary Tables S2-S3).
3.2 One-year net survival trends
Age-standardized 1-year net survival increased substantially between 2001-2003 and 2009-2014, from 25.6% (95% confidence interval [CI], 25.3%-26.0%) to 34.7% (34.5%-35.0%) (Table 2). The increase was 10%-12% for patients diagnosed at a localized or regional stage, reaching 59.8% (59.1%-60.5%) for localized disease and 53.0% (52.5%-53.4%) for regional disease. The increase was smaller (6%-7%) for patients with distant disease, reaching 19.8% (19.5%-20.1%), and for patients with unknown stage, reaching 28.9% (27.9%-29.9%). The overall increases were similar among men (from 24.6% to 33.5%) and women (from 27.1% to 36.4%). Comparable results were found for patients with unknown stage (men: from 25.1% to 27.9%; women: from 28.0% to 30.7%).
| 1-year net survival [% (95% confidence interval)] | |||||
|---|---|---|---|---|---|
| Population | Variable | Calendar period | All races | Whites | Blacks |
| The whole cohort | Total | 2001-2003 | 25.6 (25.3-26.0) | 26.1 (25.7-26.4) | 22.1 (21.2-23.0) |
| 2004-2008 | 28.6 (28.4-28.9) | 29.1 (28.8-29.4) | 24.9 (24.3-25.6) | ||
| 2009-2014 | 34.7 (34.5-35.0) | 35.1 (34.8-35.3) | 31.4 (30.8-32.0) | ||
| Stage at diagnosis | |||||
| Localized | 2001-2003 | 49.2 (48.0-50.5) | 49.6 (48.2-51.0) | 44.6 (40.9-48.3) | |
| 2004-2008 | 53.0 (52.1-53.9) | 53.6 (52.7-54.6) | 47.6 (45.2-49.9) | ||
| 2009-2014 | 59.8 (59.1-60.5) | 60.1 (59.3-60.9) | 55.3 (53.3-57.3) | ||
| Regional | 2001-2003 | 41.2 (40.5-41.9) | 41.8 (41.1-42.6) | 36.4 (34.5-38.4) | |
| 2004-2008 | 46.2 (45.7-46.7) | 46.8 (46.2-47.3) | 41.1 (39.7-42.6) | ||
| 2009-2014 | 53.0 (52.5-53.4) | 53.3 (52.8-53.8) | 49.8 (48.6-51.1) | ||
| Distant | 2001-2003 | 13.3 (12.9-13.7) | 13.6 (13.2-14.0) | 10.7 (9.7-11.6) | |
| 2004-2008 | 15.7 (15.4-16.0) | 16.0 (15.7-16.3) | 13.3 (12.6-14.0) | ||
| 2009-2014 | 19.8 (19.5-20.1) | 20.1 (19.8-20.5) | 17.1 (16.3-17.8) | ||
| Unknown | 2001-2003 | 26.3 (25.3-27.3) | 26.6 (25.5-27.8) | 23.7 (21.1-26.2) | |
| 2004-2008 | 26.6 (25.7-27.5) | 26.6 (25.6-27.6) | 25.0 (22.8-27.3) | ||
| 2009-2014 | 28.9 (27.9-29.9) | 28.5 (27.4-29.7) | 28.8 (26.4-31.1) | ||
| Men | Total | 2001-2003 | 24.6 (24.2-25.1) | 25.2 (24.7-25.7) | 19.6 (18.3-20.9) |
| 2004-2008 | 27.4 (27.1-27.8) | 28.1 (27.7-28.4) | 22.2 (21.3-23.1) | ||
| 2009-2014 | 33.5 (33.2-33.9) | 34.1 (33.7-34.4) | 28.6 (27.7-29.5) | ||
| Stage at diagnosis | |||||
| Localized | 2001-2003 | 49.0 (47.1-50.9) | 49.2 (47.2-51.3) | 43.0 (37.6-48.4) | |
| 2004-2008 | 51.7 (50.4-53.0) | 52.8 (51.4-54.2) | 41.9 (38.2-45.5) | ||
| 2009-2014 | 59.3 (58.2-60.4) | 59.8 (58.6-61.0) | 53.5 (50.3-56.7) | ||
| Regional | 2001-2003 | 41.2 (40.2-42.2) | 41.8 (40.7-42.8) | 35.4 (32.4-38.5) | |
| 2004-2008 | 45.9 (45.2-46.6) | 46.5 (45.8-47.3) | 39.7 (37.6-41.9) | ||
| 2009-2014 | 53.0 (52.4-53.7) | 53.4 (52.7-54.1) | 48.9 (46.9-50.8) | ||
| Distant | 2001-2003 | 12.7 (12.2-13.1) | 13.1 (12.6-13.6) | 9.1 (7.8-10.4) | |
| 2004-2008 | 14.9 (14.6-15.3) | 15.4 (15.0-15.8) | 11.3 (10.3-12.2) | ||
| 2009-2014 | 18.9 (18.5-19.3) | 19.3 (18.9-19.8) | 15.0 (14.0-16.0) | ||
| Unknown | 2001-2003 | 25.1 (23.8-26.5) | 25.6 (24.1-27.1) | 20.4 (16.9-23.9) | |
| 2004-2008 | 25.5 (24.3-26.7) | 25.6 (24.3-26.9) | 23.8 (20.7-27.0) | ||
| 2009-2014 | 27.9 (26.6-29.2) | 27.7 (26.2-29.2) | 26.6 (23.3-30.0) | ||
| Women | Total | 2001-2003 | 27.1 (26.6-27.5) | 27.4 (26.8-28.0) | 24.6 (23.3-25.9) |
| 2004-2008 | 30.3 (29.9-30.7) | 30.7 (30.3-31.1) | 27.6 (26.6-28.5) | ||
| 2009-2014 | 36.4 (36.1-36.8) | 36.6 (36.2-37.0) | 34.3 (33.4-35.2) | ||
| Stage at diagnosis | |||||
| Localized | 2001-2003 | 49.6 (47.9-51.4) | 50.2 (48.2-52.1) | 44.5 (39.5-49.5) | |
| 2004-2008 | 54.4 (53.2-55.6) | 54.6 (53.3-56.0) | 51.7 (48.5-54.8) | ||
| 2009-2014 | 60.4 (59.4-61.4) | 60.6 (59.5-61.8) | 56.7 (54.1-59.3) | ||
| Regional | 2001-2003 | 41.6 (40.6-42.6) | 42.2 (41.1-43.3) | 37.6 (34.9-40.2) | |
| 2004-2008 | 46.7 (46.0-47.4) | 47.3 (46.5-48.1) | 42.4 (40.5-44.3) | ||
| 2009-2014 | 53.1 (52.4-53.7) | 53.3 (52.6-54.0) | 50.8 (49.1-52.4) | ||
| Distant | 2001-2003 | 14.3 (13.7-14.8) | 14.4 (13.8-15.1) | 12.7 (11.3-14.1) | |
| 2004-2008 | 16.8 (16.4-17.2) | 16.9 (16.5-17.4) | 15.4 (14.3-16.4) | ||
| 2009-2014 | 21.2 (20.7-21.6) | 21.4 (20.9-21.9) | 19.6 (18.5-20.6) | ||
| Unknown | 2001-2003 | 28.0 (26.4-29.5) | 28.0 (26.2-29.7) | 27.4 (23.9-30.9) | |
| 2004-2008 | 28.5 (27.1-29.9) | 28.5 (27.0-30.1) | 26.6 (23.3-29.9) | ||
| 2009-2014 | 30.7 (29.2-32.2) | 30.2 (28.4-32.0) | 30.9 (27.7-34.2) | ||
One-year net survival was about 4% higher in whites than in blacks (26.1% vs. 22.1% during 2001-2003; 35.1% vs. 31.4% during 2009-2014) for all states combined (Table 2) and in most states (Figure 1, Supplementary Table S4). Most of the survival estimates for blacks were lower than the pooled US value and below the lower control limits for all three calendar periods in several states, suggesting shorter net survival than would be expected by chance. The difference in 1-year net survival between blacks and whites in all three calendar periods was slightly more marked among patients with localized or regional disease than among those diagnosed at a distant stage (Table 2). The gap was wider among men than among women.
During 2009-2014, the gap in 1-year net survival between blacks and whites was 1.8% to 4.0% in each category of stage and age at diagnosis (Figure 2, Supplementary Table S5). However, for younger women with a localized tumor, the gap in 1-year net survival between blacks and whites fell conspicuously from 13.9% to 0.9% between 2001-2003 and 2009-2014.
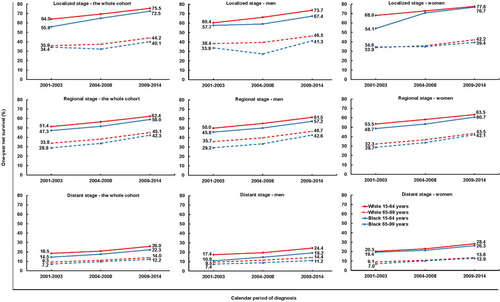
3.3 Five-year net survival trends
Five-year net survival increased from 7.0% to 11.5% among whites and from 6.8% to 10.6% among blacks (Table 3). In most states, these differences were smaller than those for 1-year net survival (Figure 3, Supplementary Table S4). Five-year net survival was 1%-3% higher among women than among men in both racial groups (Table 3).
| 5-year net survival [% (95% confidence interval)] | |||||
|---|---|---|---|---|---|
| Population | Variable | Calendar period | All races | Whites | Blacks |
| The whole cohort | Total | 2001-2003 | 7.1 (6.9-7.4) | 7.0 (6.8-7.3) | 6.8 (6.2-7.4) |
| 2004-2008 | 8.5 (8.3-8.7) | 8.5 (8.3-8.7) | 7.6 (7.2-8.1) | ||
| 2009-2014 | 11.6 (11.4-11.8) | 11.5 (11.2-11.8) | 10.6 (10.0-11.2) | ||
| Stage at diagnosis | |||||
| Localized | 2001-2003 | 24.0 (22.8-25.2) | 24.0 (22.7-25.3) | 21.3 (18.1-24.6) | |
| 2004-2008 | 30.3 (29.4-31.2) | 30.8 (29.8-31.7) | 25.4 (23.2-27.7) | ||
| 2009-2014 | 38.8 (37.8-39.8) | 39.7 (38.5-40.8) | 31.0 (28.3-33.7) | ||
| Regional | 2001-2003 | 9.4 (9.0-9.9) | 9.2 (8.7-9.7) | 9.5 (8.2-10.8) | |
| 2004-2008 | 11.7 (11.3-12.0) | 11.7 (11.3-12.1) | 10.3 (9.3-11.2) | ||
| 2009-2014 | 14.9 (14.4-15.4) | 14.8 (14.3-15.4) | 14.7 (13.4-16.0) | ||
| Distant | 2001-2003 | 2.8 (2.6-3.0) | 2.7 (2.5-2.9) | 2.7 (2.1-3.2) | |
| 2004-2008 | 3.2 (3.0-3.3) | 3.1 (2.9-3.2) | 3.1 (2.7-3.5) | ||
| 2009-2014 | 4.3 (4.1-4.6) | 4.2 (3.9-4.4) | 4.3 (3.8-4.8) | ||
| Unknown | 2001-2003 | 9.6 (8.9-10.4) | 9.3 (8.5-10.2) | 9.3 (7.5-11.2) | |
| 2004-2008 | 10.6 (9.9-11.3) | 10.6 (9.8-11.3) | 9.5 (7.8-11.2) | ||
| 2009-2014 | 13.1 (12.1-14.2) | 12.6 (11.4-13.8) | 13.4 (11.1-15.6) | ||
| Men | Total | 2001-2003 | 6.6 (6.3-6.9) | 6.5 (6.2-6.8) | 6.2 (5.4-7.0) |
| 2004-2008 | 7.9 (7.6-8.1) | 7.9 (7.7-8.2) | 6.5 (5.8-7.1) | ||
| 2009-2014 | 10.9 (10.5-11.2) | 10.9 (10.5-11.3) | 9.1 (8.2-9.9) | ||
| Stage at diagnosis | |||||
| Localized | 2001-2003 | 22.7 (20.9-24.5) | 23.2 (21.3-25.1) | 18.3 (13.8-22.9) | |
| 2004-2008 | 28.0 (26.7-29.3) | 29.2 (27.8-30.7) | 19.3 (16.0-22.5) | ||
| 2009-2014 | 38.3 (36.8-39.8) | 39.1 (37.5-40.7) | 28.5 (24.3-32.8) | ||
| Regional | 2001-2003 | 9.4 (8.7-10.0) | 9.1 (8.4-9.8) | 10.0 (7.9-12.1) | |
| 2004-2008 | 11.5 (11.0-12.0) | 11.6 (11.0-12.1) | 9.5 (8.1-10.9) | ||
| 2009-2014 | 14.6 (13.9-15.3) | 14.4 (13.6-15.2) | 14.6 (12.7-16.6) | ||
| Distant | 2001-2003 | 2.6 (2.4-2.9) | 2.5 (2.3-2.8) | 2.5 (1.8-3.2) | |
| 2004-2008 | 3.0 (2.8-3.2) | 3.0 (2.8-3.2) | 2.8 (2.2-3.4) | ||
| 2009-2014 | 4.1 (3.8-4.3) | 4.0 (3.7-4.4) | 3.2 (2.6-3.9) | ||
| Unknown | 2001-2003 | 8.5 (7.5-9.4) | 8.3 (7.2-9.4) | 7.2 (4.8-9.5) | |
| 2004-2008 | 9.9 (9.0-10.8) | 9.9 (8.9-10.9) | 8.8 (6.5-11.2) | ||
| 2009-2014 | 12.6 (11.2-14.0) | 12.4 (10.8-14.0) | 11.3 (8.4-14.2) | ||
| Women | Total | 2001-2003 | 7.9 (7.5-8.2) | 7.7 (7.4-8.1) | 7.5 (6.7-8.4) |
| 2004-2008 | 9.4 (9.1-9.7) | 9.3 (9.0-9.6) | 8.9 (8.2-9.5) | ||
| 2009-2014 | 12.6 (12.2-12.9) | 12.4 (12.0-12.8) | 12.1 (11.2-12.9) | ||
| Stage at diagnosis | |||||
| Localized | 2001-2003 | 25.2 (23.6-26.9) | 24.8 (22.9-26.6) | 23.9 (19.5-28.4) | |
| 2004-2008 | 32.6 (31.3-33.8) | 32.4 (31.0-33.7) | 30.6 (27.6-33.6) | ||
| 2009-2014 | 39.4 (38.0-40.8) | 40.4 (38.9-42.0) | 32.8 (29.3-36.3) | ||
| Regional | 2001-2003 | 9.7 (9.1-10.4) | 9.6 (8.8-10.3) | 9.3 (7.6-11.1) | |
| 2004-2008 | 12.0 (11.4-12.5) | 11.9 (11.4-12.5) | 10.8 (9.5-12.1) | ||
| 2009-2014 | 15.3 (14.6-16.0) | 15.4 (14.6-16.2) | 14.7 (13.0-16.5) | ||
| Distant | 2001-2003 | 3.0 (2.7-3.3) | 2.9 (2.5-3.2) | 2.8 (2.1-3.6) | |
| 2004-2008 | 3.4 (3.2-3.7) | 3.2 (3.0-3.5) | 3.4 (2.8-4.0) | ||
| 2009-2014 | 4.8 (4.4-5.1) | 4.4 (4.0-4.8) | 5.5 (4.6-6.3) | ||
| Unknown | 2001-2003 | 11.1 (9.9-12.4) | 10.7 (9.3-12.0) | 11.8 (9.2-14.3) | |
| 2004-2008 | 11.8 (10.7-12.9) | 11.9 (10.6-13.1) | 10.4 (7.9-12.8) | ||
| 2009-2014 | 14.1 (12.5-15.6) | 13.0 (11.2-14.9) | 15.3 (12.3-18.2) | ||
For patients diagnosed during 2009-2014, 5-year net survival reached 38.8% for localized disease and 14.9% for regional disease, but only 4.3% among patients diagnosed at a distant stage (Table 3), who comprised 50.7% of all patients (Supplementary Table S2). During 2001-2014, 5-year net survival increased by 9%-16% for localized disease and 5%-6% for regional disease, but very little for distant disease (1%-3%). Survival for patients with unknown stage also rose by 2%-4%, reaching about 13% in both racial groups, similar to that for regional disease (Table 3).
For localized tumors, 5-year net survival increased by up to 20.5% among younger patients and by 5%-10% among older patients of both sexes (Figure 4, Supplementary Table S6). The increases were more marked for whites than for blacks: the racial difference widened from 2.7% (24.0% vs. 21.3%) during 2001-2003 to 8.7% (39.7% vs. 31.0%) during 2009-2014 (Table 3).
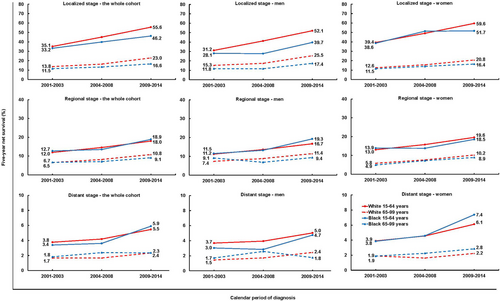
Trends in 5-year net survival from pancreatic cancer among US adults (15-99 years) during 2001-2014 stratified by age, sex, race and stage.
The y axis scales are different for localized disease (0-80%), regional disease (0-40%) and distant disease (0-10%).
During 2009-2014, the racial gap in 5-year net survival from localized tumors was 8.1%-12.4% among men and 4.4%-7.9% among women (Figure 4, Supplementary Table S6).
4 DISCUSSION
This study estimates the trends in population-based survival among US adults diagnosed with invasive pancreatic cancer during 2001-2014, stratified by age, sex, race and stage at diagnosis, with data from cancer registries in 41 states that include 85% of the US population. Survival increased over time, especially for patients diagnosed with a localized tumor, but gaps in 1- and 5-year survival between blacks and whites were persistent throughout this 14-year period.
The increase in survival may be partly explained by the adoption of better diagnostic techniques and more effective treatment. Since its first use in the early 1990s, the accuracy of ultrasound-guided endoscopic fine-needle aspiration for diagnosing pancreatic cancer has reached a sensitivity of 87% and a specificity of 96% [27], with improved staging, more effective treatment and better survival.
Because surgery is currently the only treatment modality to offer any prospect of cure, the definition of resectable pancreatic cancer is crucial. This definition, first developed in 2009 [28], has evolved [29]. No single definition has been agreed, but the criteria underpinning these definitions are designed to improve the selection of patients for surgery, thus increasing the likelihood of surgical resection with margins that are clear of tumor. Differences between blacks and whites in stage-specific survival were widest for both younger and older patients diagnosed at a localized stage, i.e., those stages for which surgery with adjuvant chemotherapy is crucial [6]. More limited access to health care and treatment is likely to be an important reason behind the survival deficit among blacks.
Two federally funded health insurance programs are in operation in the US, Medicare and Medicaid. Medicare provides health insurance to people aged 65 and older, and Medicaid to persons with limited income and resources. For other people, the availability of private health insurance is essential for access to health care and treatment. In general, blacks are more likely than whites to present for diagnosis without any medical insurance or with Medicaid insurance [30, 31]. As a consequence, blacks are less likely than whites to receive surgery [32, 33]. The lack of private medical insurance may therefore limit access to specialist health centers that can provide state-of-the-art treatment. Similar conclusions have been reached from survival analyses by insurance status, using data from the SEER program for adults (18-64 years) diagnosed during 2007-2010 with one of the ten most common cancers, including pancreatic cancer [30]. Lack of health insurance was associated with non-white race and the male sex. Patients with Medicaid coverage or without health insurance were more likely to present with advanced disease, less likely to receive cancer-directed surgery or radiotherapy, and more likely to experience worse survival.
By contrast, in health care systems that provide equal access to treatment, regardless of race, the evidence suggests that there are no important disparities in treatment, or survival, between white and black patients with pancreatic cancer [18, 34]. For example, the Department of Defense tumor registry was used to study 1,008 patients diagnosed with pancreatic adenocarcinoma between 1993 and 2007; 15.6% of the patients were black. Tumors were diagnosed at loco-regional stage among 36.3% of blacks and 37.3% of whites. There was no evidence of differences between blacks and whites in the odds of receiving surgery, chemotherapy or radiotherapy, and overall survival was not shorter among blacks than among whites [34]. A more recent cohort study included adults diagnosed with pancreatic ductal adenocarcinoma between 2006 and 2014 within the Kaiser Permanente Southern California [18], i.e., the combination of Kaiser Foundation Hospitals and Kaiser Foundation Health Plans, which are committed to providing programs that facilitate access to care for vulnerable populations. Minorities were not found to be disadvantaged in pancreatic cancer care, and the hazard of death from pancreatic cancer was around 20% lower among non-Hispanic blacks than among non-Hispanic whites (hazard ratio = 0.78, 95% CI = 0.67-0.91).
We found that the survival gap between black and white patients in the general US population existed among both younger patients (15-64 years), many of whom would be covered by private health insurance, and older patients, who are almost all covered by Medicare. However, the gap was somewhat smaller among older than younger patients. This underlines the complexity of the issue. It suggests that the black-white survival gap could be reduced if young black patients had greater access to health insurance and that other factors may contribute to this survival gap, such as a decision to decline surgery or other treatment and the surgical caseload volume of the hospital. In one population-based study using SEER data on 45,509 patients (10.9% African-American) diagnosed with a histologically confirmed adenocarcinoma of the pancreas between 1983 and 2007, black race was one of the factors predictive of the decision to refuse surgery (odds ratio = 1.53, 95% CI = 1.14–2.04) [35]. Another population-based study using SEER data on 35,944 patients diagnosed with potentially resectable pancreatic cancer during 1988-2009 showed that black patients were more likely to refuse surgery when it was recommended as the therapy of choice [36].
A study of patients discharged from New York City area hospitals following cancer surgery during 2001-2004 showed that, even after adjustment for age, sex, income level, type of health insurance, comorbidity and proximity to a high-volume hospital, blacks with pancreatic cancer were significantly more likely to have been treated by low-volume surgeons or at hospitals with lower surgical caseload, both of which are factors associated with outcome [37].
The gap in survival between blacks and whites is unlikely to be explained by differences in the proportions of ductal pancreatic adenocarcinomas, which comprise 80% or more of pancreatic cancers and have a poor prognosis, and neuroendocrine tumors, which are much less common but have better survival [6]. A population-based study of 57,688 patients (12.1% blacks) diagnosed with pancreatic cancer during 2004-2012 in 18 SEER cancer registries showed no substantial difference between whites and blacks in the proportion of adenocarcinomas (93.2% vs. 90.9%) or neuroendocrine tumors (6.8% vs. 8.1%) [8]. Survival for blacks with adenocarcinoma was also lower than for whites after controlling for age at diagnosis, period of diagnosis, sex, stage, morphology, grade and anatomical site [8].
Our results show that blacks were more likely than whites to be diagnosed at a distant stage, especially men. Survival was slightly higher for women than for men, among both blacks and whites. These results are compatible with the findings that non-whites [38] and men [39] may have lower awareness of cancer symptoms than whites and women, respectively, which could contribute to delay in diagnosis and a survival advantage for women over men.
Our results on net survival are consistent with those in the most recent edition of Cancer in North America [40]: age-standardized 5-year relative survival for patients diagnosed during 2008-2014 was similar among whites (11.0%) and blacks (10.0%), with a small difference among men (10.4% for whites vs. 8.2% for blacks), but not for women (11.7% vs. 11.9%). Five-year survival estimates by stage were 38.3% for localized, 14.0% for regional and 3.8% for distant stage, which were closely similar to those we reported for 2009-2014 (38.8%, 14.9%, and 4.3%, respectively).
Although this study focused on patients diagnosed in 2001-2014, it is one of the largest population-based studies of pancreatic cancer survival, including data from 41 states with 85% of the US population. It provides the most up-to-date complete picture of the distribution of stage at diagnosis and stage-specific survival among blacks and whites, stratified by age and sex. We were not able to produce reliable estimates of survival for other ethnic and racial minorities, including Asian or Pacific Islander, American Indian/Alaska Native or other unspecified or unknown races, because they only comprised 4% of the cases, and robust life tables to correct for background mortality for these groups were not available. The CONCORD-3 database includes essential data collected by population-based cancer registries (e.g., demographic data, anatomic site and morphology of the primary tumor, stage at diagnosis, vital status, and the date of death when the patient has died). The database does not include data on comorbidities or risk factors that may also be prognostic factors, which can help interpret survival differences. Socioeconomic status is related to race/ethnicity in the US, but the ability of socioeconomic status to explain differences within ethnic groups is limited [41].
5 CONCLUSIONS
This study confirms a persistent disparity in 1- and 5-year net survival from pancreatic cancer between blacks and whites diagnosed in the US during the period 2001-2014. One-year net survival has improved considerably, but the black-white disparity remains unchanged, either for all stages combined or by stage at diagnosis. Five-year net survival has also improved, but there are also persistent differences between blacks and whites, especially for patients with localized tumors, for which surgery is currently the only treatment modality with the potential for cure. This pattern suggests that differences in access to health insurance, high-quality resection and other treatment modalities may be more important than comorbidity or socioeconomic status in explaining the persistent disparities in survival between blacks and whites. Improving these aspects of access to health care would be expected to reduce the black-white disparities in pancreatic cancer survival, especially for those with localized disease.
DECLARATIONS
AUTHOR CONTRIBUTIONS
Study design: CA and MPC. Acquisition of statutory and ethical approvals: MPC and CA. Data quality control: PM, MN, CA, MPC. Formal analyses: PM, MN. Writing original draft: MN, PM, CA, MPC. All authors, including all members of the US CONCORD Working Group, made substantial contributions to the acquisition, preparation, quality control and analysis of the data, and contributed to interpretation of the findings. Review and editing: All authors checked and critically revised the original draft for important intellectual content, and contributed to writing and approved the final report. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Funding acquisition: CA and MPC.
ACKNOWLEDGMENTS
Source of funding
We thank the Centers for Disease Control and Prevention, US National Cancer Institute and the American Cancer Society for supporting this project.
Role of the funding source
The funding sources played no part in the design, data collection, quality control, analysis, interpretation of the findings, manuscript writing or the decision to submit for publication. The corresponding author had full access to all the data and responsibility for submission for publication.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention, US National Cancer Institute or the American Cancer Society.
DECLARATION OF COMPETING INTEREST
The authors have no conflicts of interest to declare.
DATA SHARING AND DATA AVAILABILITY
The data underlying this article cannot be shared because they are personal data, provided in anonymized form by participating US cancer registries to the CONCORD program under relevant ethical and statutory approvals in the United States and the United Kingdom to protect the privacy of individuals. Requests for data should be addressed to the registry or registries concerned.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The Cancer Survival Group maintains approval for processing sensitive personal data for the CONCORD program from the UK's statutory Health Research Authority (reference ECC 3-04(i)/2011; last update, October 17, 2022), the National Health Service Research Ethics Service (11/LO/0331; November 2, 2022), and the Ethics Committee of the London School of Hygiene & Tropical Medicine (12171; September 1, 2022).
CONSENT FOR PUBLICATION
Consent for publication is not applicable because the data are anonymized by the source registries.
US CONCORD WORKING GROUP
America (North)—USA: T Freeman, JT George (Alabama Statewide Cancer Registry); RM Avila, DK O'Brien (Alaska Cancer Registry); A Holt (Arkansas Central Cancer Registry); L Almon (Metropolitan Atlanta Registry); S Kwong, C Morris (California State Cancer Registry); R Rycroft (Colorado Central Cancer Registry); L Mueller, CE Phillips (Connecticut Tumor Registry); H Brown, B Cromartie (Delaware Cancer Registry); J Ruterbusch, AG Schwartz (Metropolitan Detroit Cancer Surveillance System); GM Levin, B Wohler (Florida Cancer Data System); R Bayakly (Georgia Cancer Registry); KC Ward (Georgia Cancer Registry; Metropolitan Atlanta Registry); SL Gomez, M McKinley (Greater Bay Area Cancer Registry); R Cress (Cancer Registry of Greater California); J Davis, B Hernandez (Hawaii Tumor Registry); CJ Johnson, BM Morawski (Cancer Data Registry of Idaho); LP Ruppert (Indiana State Cancer Registry); S Bentler, ME Charlton (State Health Registry of Iowa); B Huang, TC Tucker* (Kentucky Cancer Registry); D Deapen, L Liu (Los Angeles Cancer Surveillance Program); MC Hsieh, XC Wu (Louisiana Tumor Registry); M Schwenn (Maine Cancer Registry); K Stern (Maryland Cancer Registry); ST Gershman, RC Knowlton (Massachusetts Cancer Registry); G Alverson, T Weaver (Michigan State Cancer Surveillance Program); J Desai (Minnesota Cancer Reporting System); DB Rogers (Mississippi Cancer Registry); J Jackson-Thompson (Missouri Cancer Registry and Research Center); D Lemons, HJ Zimmerman (Montana Central Tumor Registry); M Hood, J Roberts-Johnson (Nebraska Cancer Registry); W Hammond, JR Rees (New Hampshire State Cancer Registry); KS Pawlish, A Stroup (New Jersey State Cancer Registry); C Key, C Wiggins (New Mexico Tumor Registry); AR Kahn, MJ Schymura (New York State Cancer Registry); S Radhakrishnan, C Rao (North Carolina Central Cancer Registry); LK Giljahn, RM Slocumb (Ohio Cancer Incidence Surveillance System); C Dabbs, RE Espinoza (Oklahoma Central Cancer Registry); KG Aird, T Beran (Oregon State Cancer Registry); JJ Rubertone, SJ Slack (Pennsylvania Cancer Registry); J Oh (Rhode Island Cancer Registry); TA Janes, SM Schwartz (Seattle Cancer Surveillance System); SC Chiodini, DM Hurley (South Carolina Central Cancer Registry); MA Whiteside (Tennessee Cancer Registry); S Rai, MA Williams (Texas Cancer Registry); K Herget, C Sweeney (Utah Cancer Registry); J Kachajian (Vermont Cancer Registry); MB Keitheri Cheteri, P Migliore Santiago (Washington State Cancer Registry); SE Blankenship, JL Conaway (West Virginia Cancer Registry); R Borchers, R Malicki (Wisconsin Department of Health Services); J Espinoza, J Grandpre (Wyoming Cancer Surveillance Program); HK Weir*, R Wilson (Centers for Disease Control and Prevention); BK Edwards*, A Mariotto (National Cancer Institute); C Rodriguez-Galindo* (St. Jude Children's Research Hospital)
*CONCORD Steering Committee




