Treatment intensification for metastatic prostate cancer: New treatment landscapes in androgen deprivation-based therapy
List of abbreviations
-
- mPC
-
- metastatic prostate cancer
-
- mCRPC
-
- metastatic castration resistant prostate cancer
-
- ARTA
-
- androgen receptor-targeting agent
-
- ADT
-
- androgen deprivation therapy
-
- OS
-
- overall survival
-
- mHSPC
-
- metastatic hormone-sensitive prostate cancer
-
- HR
-
- hazard ratio
-
- CI
-
- confidence interval
-
- ASCO GU
-
- American Society of Clinical Oncology Genitourinary
-
- Ra-223
-
- radium-223
-
- Lu-PSMA
-
- Lutetium-177 - PSMA-617
-
- PSMA
-
- prostate-specific membrane antigen
-
- PARPi
-
- poly ADP-ribose polymerase inhibitors inhibitor
-
- DDR
-
- DNA damage repair
-
- HRR
-
- homologous recombination repair
-
- BRCA
-
- Breast Related Cancer Antigens
-
- rPFS
-
- radiological progression-free survival
-
- PET
-
- positron emission tomography
-
- PFS
-
- progression free survival
-
- PFS2
-
- progression free survival with first subsequent therapy
1 BACKGROUND
Since 2004, the treatment landscape of metastatic prostate cancer (mPC) has significantly changed. When added to androgen deprivation therapy (ADT), many treatments demonstrated to improve overall survival (OS) of mPC patients both in hormone-sensitive (mHSPC) [1] and castration-resistant (mCRPC) setting [2]. With multiple available options, therapy selection and the optimal treatment sequence are currently crucial clinical challenges.
Recently, at the American Society of Clinical Oncology Genitourinary 2022 (ASCO GU 2022) congress, the results of large trials were presented that could once again change the mPC therapeutic landscape. Our aim was to describe therapies currently available in metastatic setting, to analyze the data of the potentially practice-changing studies presented at ASCO GU 2022, and to discuss the possible future therapeutic scenarios in mPC management.
2 TREATMENT OF MPC: STATE OF THE ART
The landscape of mHSPC has significantly changed over the past decade. Since 2015, new treatment approaches using docetaxel or androgen receptor-targeting agents (ARTAs) (abiraterone, enzalutamide and apalutamide) in combination with ADT have resulted in significantly improved survival rates compared to ADT alone [1]. Primary tumor irradiation in addition to ADT has also been shown to improve OS in patients with low-volume mHSPC according to the CHAARTED criteria [3, 4].
One of the most relevant clinical characteristics that can help physicians in selecting the best treatment for mHSPC patients is the disease volume according to the CHAARTED criteria. Current guidelines recommend docetaxel use preferably in patients with high-volume mHSPC [5], while local radiotherapy can only be considered in low-volume patients [5, 6]. Abiraterone, enzalutamide and apalutamide, on the other hand, can be used in both low- and high-volume patients [4, 5].
In mCRPC patients, several treatments have been shown to be effective [2]. However, a clear therapeutic sequence has not been identified. Docetaxel, abiraterone, and enzalutamide have all shown significant survival benefits as first-line therapies [2]. Cabazitaxel, abiraterone and enzalutamide showed efficacy in mCRPC patients progressed after docetaxel treatment [2]. In the CARD trial, cabazitaxel improved OS in mCRPC setting as a third-line treatment in patients who had previously received docetaxel and an ARTA (abiraterone or enzalutamide) [2]. Several studies demonstrated that the sequential use of ARTAs is not very effective due to cross-resistance mechanisms [7, 8]. For this reason, the ARTA-ARTA sequence should be used only in very selected cases.
Radium-223 (Ra-223) is an alpha targeted emitter that binds to areas of increased turnover in skeletal metastases. This drug improved OS in bone metastatic mCRPC patients [2]. According to European Medicines Agency, Ra-223 should only be used in patients who have previously received docetaxel and ARTAs [9]. This recommendation is based on the results of the ERA-223 trial in which Ra-223 + abiraterone showed an increased rate of fractures when compared to abiraterone alone [10]. Lutetium-177-PSMA-617 (Lu-PSMA) is a radiolabelled small molecule that delivers β radiation to cells expressing prostate-specific membrane antigen (PSMA). In the VISION trial, Lu-PSMA showed OS improvement in patients previously treated with at least one ARTA and one or two taxane regimens [11]. Olaparib, a poly(ADP-ribose) polymerase inhibitor (PARPi) acting through synthetic lethality in the presence of mutations in DNA damage repair (DDR) genes, improved OS in patients previously treated with abiraterone or enzalutamide [2].
3 NEW DATA FROM ASCO GU 2022
Both ARTA + ADT and docetaxel + ADT prolong OS in mHSPC patients, and potentially triplet therapy (ADT + ARTA + docetaxel) could further improve disease outcome. However, the primary results of the efficacy of the triplet therapy were disappointing. Patients receiving triplet therapy in the TITAN [12], ARCHES [13] and ENZAMET trials [14] had no benefit from the addition of docetaxel to ADT + ARTA.
The most recent data from the PEACE-1 trial [15], evaluating ADT + docetaxel + abiraterone (+/- local radiotherapy) versus ADT + docetaxel (+/- local radiotherapy) in mHSPC patients, demonstrated a significant improvement in OS achieved by triplet therapy especially in patients with high-volume disease.
Considering these conflicting data, the results of the phase III ARASENS study were largely awaited. In this trial, mHSPC patients were randomized to receive either ADT + docetaxel + darolutamide or ADT + docetaxel + placebo, and the final results were presented at the ASCO GU 2022 Congress. The primary endpoint of OS was met, and the risk of death was significantly lower in the darolutamide group [hazard ratio (HR) = 0.68, 95% confidence interval (CI) = 0.57-0.80, P < 0.001]. Based on these results, triplet therapy should be considered the new standard of care for mHSPC patients [16].
However, the ARASENS trial raised some crucial questions. One concern is the identification of patients benefiting most from triplet therapy in order to avoid the adverse events and high costs of ARTAs in patients who are highly responsive to ADT + docetaxel. The PEACE-1 study showed the greatest efficacy of triplet therapy in high-volume mHSPC patients [15]. The outcomes of patients with different disease volumes are not available from the ARASENS study, and this information would be useful to know if triplet therapy can be avoided in patients with low-volume disease. PEACE-1 [15] and ARASENS studies [16] demonstrated that triplet therapy improved outcomes compared to ADT + docetaxel. However, there is currently no evidence that triplet therapy is better than ADT + ARTA. The identification of patients who do not need chemotherapy is crucial because triplet therapy toxicity is mainly docetaxel-related.
In the mCRPC setting, the most interesting data presented at ASCO GU 2022 concerned two phase III studies that evaluated the combination of PARPi and ARTA [17, 18]. PARP inhibition increases activity of ARTAs via androgen receptor-dependent transcription, and ARTAs induced homologous recombination repair (HRR) deficiency, increasing the susceptibility to PARPi and providing a strong pre-clinical rationale to test this combination [17, 18].
In the MAGNITUDE trial [17], the combination of niraparib and abiraterone (experimental arm) versus abiraterone alone was tested in patients with and without DDR gene alterations. In patients without alterations, there was no benefit from the experimental arm; while in patients with DDR mutations, niraparib + abiraterone resulted in a statistically significant improvement in radiological progression-free survival (rPFS) (16.5 months vs. 13.7 months, HR = 0.73, 95% CI = 0.56-0.96, P = 0.0217). In the PROPEL study [18], olaparib + abiraterone improved rPFS compared to abiraterone alone (27.6 vs. 16.4 months, HR = 0.61, 95% CI = 0.49-0.74, P < 0.0001) irrespective of HRR status. Pending mature OS results from these 2 studies, PARPi + abiraterone may be considered as first-line treatment for mCRPC patients with DDR mutations. It remains to be clarified whether all patients with DDR mutations or only those with specific mutations, such as breast-related cancer antigens (BRCA)1-2, may benefit from this combination.
4 HOW THE THERAPEUTIC SEQUENCE CHANGES AFTER ASCO GU 2022: FOUR POSSIBLE SCENARIOS
The results of the studies presented at ASCO GU 2022 meeting pave the way to four possible scenarios of therapeutic sequence in mPC patients (Figure 1).
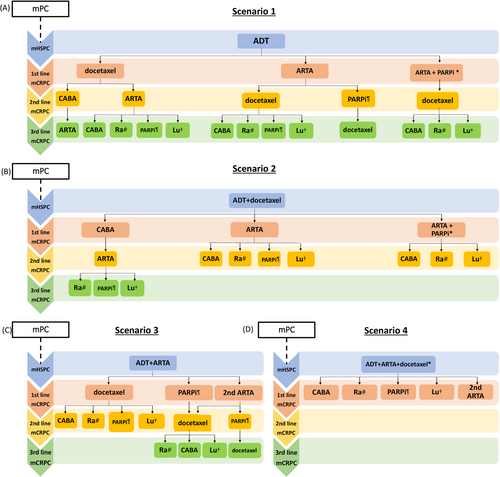
Proposal for a therapeutic algorithm in metastatic prostate cancer. (A) scenario 1: ADT in the hormone-sensitive phase; (B) scenario 2: ADT + docetaxelin the hormone-sensitive phase; (C): scenario 3: ADT + ARTA in the hormone-sensitive phase; (D) scenario 4: ADT + ARTA + docetaxel in the hormone-sensitive phase.
*Off-label use.
#Only in symptomatic patients with exclusively bone metastases.
¶Only in patients with mutations in DNA damage repair genes.
†Off-label use only in patients with positron emission tomography-prostate-specific membrane antigen (PET-PSMA)-positive disease.
Abbreviations:
mPC: metastatic prostate cancer
ADT: androgen deprivation therapy
mHSPC: metastatic hormone-sensitive prostate cancer
mCRPC: metastatic castration-resistant prostate cancer
ARTA: androgen receptor-targeting agent
PARPi: poly(ADP-ribose) polymerase inhibitor
CABA: cabazitaxel
Ra: radium 223
Lu: lutetium-177-PSMA-617
4.1 First scenario: ADT alone in the hormone-sensitive phase
According to the current guidelines, ADT monotherapy is no longer the standard of care for mHSPC and should be abandoned [5, 6]. However, to date some patients are still undergoing ADT monotherapy especially in those countries where the reimbursement systems do not allow the use of ARTAs in this setting. In patients progressing after ADT monotherapy, treatment with docetaxel or ARTAs may be considered, and abiraterone + PARPi may be added if a DDR gene mutation is detected. Patients receiving abiraterone + PARPi as first-line treatment in the mCRPC setting should receive second-line treatment with docetaxel while cabazitaxel, Ra-223 and Lu-PSMA should be used in subsequent lines.
4.2 Second scenario: ADT + docetaxel in the hormone-sensitive phase
The significant OS benefit from abiraterone or enzalutamide in patients progressed after docetaxel [2] support the hypothesis that ARTA is a reasonable first-line treatment in the mCRPC setting in patients progressed after docetaxel in the mHSPC phase. Cabazitaxel may, alternatively, be an option in patients with unfavorable prognostic factors. In patients with DDR mutations, abiraterone + PARPi as first-line mCRPC treatment can also be considered.
4.3 Third scenario: ADT + ARTA in the hormone-sensitive phase
Docetaxel is a doable option for the first-line mCRPC treatment of patients who have previously received ARTAs in the mHSPC phase. Cross-resistance between different ARTAs is well-known [7, 8], and the ARTA-ARTA sequence is not recommended by the guidelines [5, 6]. However, this evidence comes from studies in mCRPC patients and is limited to the sequence of enzalutamide and abiraterone and vice versa, while recent data in patients treated with apalutamide in earlier stages of the disease have suggested that the sequential use of ARTAs could be considered a possible therapeutic option [19, 20].
Olaparib may be another therapeutic option in patients with DDR mutations treated with ADT + ARTA in mHSPC phase.
4.4 Fourth scenario: ADT + ARTA + docetaxel in the hormone-sensitive phase
This scenario has the weakest evidence about a possible therapeutic sequence. In our opinion, in patients who progressing after triplet therapy in the mHSPC phase, several factors should be considered in the selection of the first subsequent therapy. First, the general conditions of patients are crucial. Cabazitaxel could be considered a viable option in patients with good performance status. Moreover, the disease characteristics should be considered: in patients with symptomatic bone-only disease, treatment with Ra-223 might be a viable option; in patients with DDR mutations, olaparib could represent the first choice while patients with high PSMA expression on positron emission tomography (PET)-PSMA could benefit from Lu-PSMA treatment. Finally, in the PEACE-1 [15] and ARASENS trials [16], more than 45% of patients treated with triplet therapy received at least a second ARTA in the therapeutic sequence, therefore treatment with ARTAs could also be considered in these patients, especially in asymptomatic patients with mildly progressive disease.
5 DISCUSSION
Currently, we have seven therapies for mCRPC and four for mHSPC that have been shown to prolong OS in addition to ADT [1, 2]. The results of the recent studies presented at ASCO GU 2022 could propose new therapeutic options. The ARASENS study recommended triplet therapy as the new standard of care for mHSPC patients [16]. However, several open questions remain, including which patients would benefit most from triplet therapy and whether triplet therapy is better than doublet therapy with ADT + ARTA. The results of the MAGNITUDE [17] and PROPEL trials [18] suggest that the combination of PARPi and abiraterone may represent a possible therapeutic option in first-line mCRPC patients with DDR deficiency. However, mature OS data are needed.
In this article, we described the possible therapeutic scenarios in mPC patients. However, the therapeutic sequence may vary by the treatments received in non-metastatic setting. ADT + ARTA (enzalutamide, apalutamide or darolutamide) is the standard of care for non-metastatic CRPC (nmCRPC) patients with prostate-specific antigen doubling time <10 months, and abiraterone has also been included in current guidelines for the treatment of patients with high-risk localized disease defined according to the STAMPEDE trial criteria [5, 6]. We have no evidence on the best first subsequent therapy in patients receiving ARTAs in non-metastatic setting. Considering the well-known cross-resistance among ARTAs [7, 8], docetaxel should be considered the first choice, while olaparib could be a viable option for patients with DDR mutations. However, it is crucial to consider that in nmCRPC trials a significant portion of patients received an ARTA (abiraterone or enzalutamide) as the first subsequent therapy [2]. Therefore, in patients treated with ARTAs in the nmCRPC setting, a second ARTA as the first-line therapy in the mCRPC setting could be considered a possible therapeutic option.
The results presented at the ASCO GU 2022 Congress introduced a new standard of care in the mHSPC setting and a new possible therapeutic option in the mCRPC phase, once again revolutionizing the ideal therapeutic sequence in these patients. In this complex and crowded therapeutic landscape, future studies are urgently needed to identify predictive biomarkers for selecting the best drug at the right time for the right patient.
DECLARATIONS
ACKNOWLEDGMENTS
None
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable
CONSENT FOR PUBLICATION
Not applicable
CONFLICT OF INTERESTS
None
AUTHORS' CONTRIBUTIONS
Conceptualization: Fabio Turco, Marcello Tucci, Consuelo Buttigliero, Giorgio Vittorio Scagliotti
Methodology: Fabio Turco, Marcello Tucci, Consuelo Buttigliero, Giorgio Vittorio Scagliotti
Investigation: Fabio Turco, Marcello Tucci
Writing - Original Draft: All authors
Visualization: Fabio Turco
Supervision: Marcello Tucci, Consuelo Buttigliero, Giorgio Vittorio Scagliotti
Open Research
DATA AVAILABILITY STATEMENT
Not applicable




