The five major autoimmune diseases increase the risk of cancer: epidemiological data from a large-scale cohort study in China
Abstract
Background
Cancer incidence and mortality have received critical attention during the long-term management of morbidities in patients with autoimmune diseases (AIDs). This study aimed to investigate and compare the risk of cancer associated with five major AIDs in a large-scale Chinese cohort.
Methods
A total of 8,120 AID patients consecutively admitted to a national tertiary referral center in China were included and followed-up for 38,726.55 patient-years, including those with systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), Sjögren's syndrome (SS), systemic scleroderma (SSc), and idiopathic inflammatory myositis (IIM). Demographic data, cancer incidence, predilecting sites and cancer onset time were recorded and compared among the five AIDs.
Results
Four hundred and thirty (5.3%) patients developed cancer. Their median age was 57.5 years and AID duration was 79.8 months. The estimated total standardized incidence ratio (SIR) of cancer in AIDs patients was 3.37, with the highest SIR observed in IIM (4.31), followed by RA (3.99), SSc (3.77), SS (2.88) and SLE (2.58). The increased SIR of cancers in AID patients showed a female predominance (female vs. male: 3.59 vs. 2.77) and younger patient involvement (age <50 vs. ≥50 years: 4.88 vs. 3.04). Patients with SLE had increased SIRs for developing hematologic malignancies and solid tumors located in the urinary bladder, corpus uteri and cervix uteri. Patients with SS had a significantly high SIR for developing non-Hodgkin's lymphoma. Within 3 years of IIM diagnosis, 74.6% of the patients developed cancer and they had a high risk of ovarian cancer. RA was associated with a wide distribution of scancers, including non-Hodgkin's lymphoma, gynecologic, urinary tract, thyroid gland and lung cancers. SSc patients had increased SIRs for developing cervical uterine, lung, and breast cancers.
Conclusion
Patients with five major AIDs in China had an increased risk of developing cancer, with a predominance in women and younger patients, although cancer incidence, predilection sites and cancer onset time may vary greatly in each AID entity.
List of abbreviations
-
- AIDs
-
- autoimmune diseases
-
- SLE
-
- systemic erythematosus
-
- RA
-
- rheumatoid arthritis
-
- SS
-
- Sjögren's syndrome
-
- SSc
-
- systemic sclerosis
-
- IIM
-
- idiopathic inflammatory myopathies
-
- NHL
-
- non-Hodgkin's lymphoma
-
- IRRES
-
- In-patient Registration and Recoding Electronic System
-
- PUMC
-
- Peking Union Medical College
-
- ACR
-
- American College of Rheumatology
-
- EULAR
-
- European League Against Rheumatism
-
- ICD
-
- International Classification of Disease
-
- SD
-
- standard deviation
-
- IQR
-
- interquartile range
-
- NCCR
-
- National Central Cancer Registry
-
- CI
-
- confidence interval
-
- MM
-
- multiple myeloma
-
- POLR3A
-
- RNA polymerase III autoantibodies
-
- APRIL
-
- a proliferation-inducing ligand
-
- DMARDs
-
- disease-modifying anti-rheumatic drugs
1 INTRODUCTION
Cancer incidence and mortality have raised critical attention in the long-term management of patients with autoimmune diseases (AIDs) [1]. The increased cancer risk is attributed to AIDs, medications, age, sex, and other heterogeneous factors. Although there are previous studies evaluating the cancer risks of patients with certain types of AIDs, they either focused on certain types of cancer in AID patients or followed the patients within a specific number of years.
Previous studies have reported that the incidence of hematological malignancies, especially non-Hodgkin's lymphoma (NHL), was increased in patients with Sjögren's syndrome (SS) and systemic lupus erythematosus (SLE) [2, 3]. In patients with SS, lymphoid infiltration in the salivary glands was shown to be a predictor of NHL development [4]. Other AIDs, including idiopathic inflammatory myositis (IIM) and systemic scleroderma (SSc), are known to increase the risk of cancer [5-7]. Genetic alterations in anti-transcription intermediary factor 1 (TIF1) genes are involved in the pathogenesis of cancer-associated myositis [8, 9]. However, whether the overall cancer risk increases in patients with rheumatoid arthritis (RA) remains disputable. In addition, treatments such as methotrexate and tumor necrosis factor-α antagonists have been shown to have an impact on cancer risks [10-13]. Therefore, the association between cancer and AIDs is clinically complicated and the proper management of AID patients with cancers remains challenging for rheumatologists. To date, a comprehensive comparison of the epidemiological data on cancer risks, distributions, and chronological development among patients with major AIDs with long-term follow-up is warranted for a better understanding of this severe complication in AIDs.
Herein, this large-scale retrospective study was conducted in patients with five major AIDs in China with a long-term follow-up, aiming to depict the cancer incidence in patients with preexisting AIDs, to compare the characteristics of cancer sites and cancer risks among different AIDs, and to illustrate the features of the time sequence from AID diagnosis to cancer occurrence.
2 MATERIALS AND METHODS
2.1 Cohort population and data collection
Patients diagnosed with five major AIDs between January 2006 and April 2015 were included. The patients’ medical records were retrieved from the In-patient Registration and Recoding Electronic System (IRRES) database of Peking Union Medical College (PUMC) Hospital, a tertiary referral academic center in China that is dedicated to providing high-quality evidence-based therapeutic care for AID patients nationwide. All patients fulfilled the respective classification criteria (SLE: American College of Rheumatology (ACR) 1997 [14] or Systemic Lupus Erythematosus International Collaborating Clinics 2012 criteria [15]; RA: ACR 1987 [16] or ACR/European League Against Rheumatism (EULAR) 2010 [17] criteria; SS: American-European Consensus Group classification criteria 2002 [18] or ACR 2012 [19] criteria; IIM: Bohan and Peter [20] criteria; SSc: ACR 1980 [21] or ACR/EULAR 2013 [22] criteria). Patients who developed cancer before the AID diagnosis of were excluded. AID-CA patients were defined as patients with incident cancer occurrence at the time of or after AID diagnosis. The cancer diagnosis was confirmed by biopsy or sampling of surgical specimens according to the International Classification of Diseases (ICD-10) criteria [23].
Patient data, including baseline demographics, personal history, disease duration, and AID manifestations, were obtained from the IRRES. The occurrence of cancer was documented during subsequent visits or telephone follow-ups and was double-checked by two researchers. The follow-up period was defined as the interval between discharge and the recorded date of cancer occurrence, death due to noncancerous causes, or until October 1, 2020 (censoring date). For AID-CA patients, the disease duration was determined as the time interval from AID diagnosis to cancer diagnosis. Patients who were lost to follow-up were defined as those who never returned to the clinics after discharge and were unable to contact. This study was approved by the ethics review board of PUMC Hospital (S-K1599).
2.2 Statistical analysis
Continuous variables were expressed as either mean ± standard deviation (SD) or median and interquartile range (IQR), whereas categorical data were expressed as frequencies (percentages). The expected cancer rate was calculated by multiplying patient-years by cancer incidence in the general Chinese population. The estimated cancer incidence rates referred to the age-, gender-, area-, and site-specific rates reported in 2006-2015 by the National Central Cancer Registry of China (NCCR; dataset in Supplementary Table S1) [24-26]. The standardized incidence ratios (SIRs), which is the ratio of observed to expected cancers cases, of each cancer type, were calculated to compare the risk of cancer in patients with AID with that in the general Chinese population. The 95% confidence intervals (CIs) were calculated based on the Poisson distribution. The gender and site-specific SIRs by AID were plotted in heatmaps and clustered with distances determined using the Canberra method to investigate similarities among AIDs. The analyses were performed using the R software (version 3.6.3, R Foundation for Statistical Computing), adopting the packages popEpi, pheatmap, and ggplot2 [27-29].
3 RESULTS
3.1 Demographics, cancer occurrence, and SIR in the AID
A total of 10,137 AID patients from 31 provinces across mainland China were admitted to the PUMC Hospital between January 2006 and April 2015. Among them, 8,120 (80.1%) patients with follow-up data were included. For a medium follow-up time of 4.51 patient-years, cancer was identified in 430 patients (5.3%) at the time of or after the diagnosis of AID, including SLE (90/3,794; 2.4%), RA (162/1,658; 9.8%), SS (84/1,329; 6.3%), IIM (67/964; 7.0%), and SSc (27/375; 7.2%; Figure 1). The incidence of cancer was 1,110.35/100,000 patient-years in the AID patients. The median age at cancer diagnosis was 57.5 (IQR = 48.8-66.2) years. The AID-CA patients were female predominance (female : male = 4.58 : 1) with a median disease duration of 79.8 (IQR = 14.2-145.4) months (Table 1). The demographics of the excluded patients and the comparisons between the excluded and included patients are shown in Supplementary Table S2.
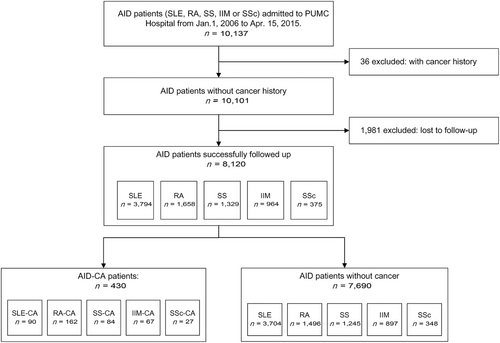
Flow chart of patient enrollment.
Abbreviations: AID, autoimmune disease; SLE, systemic lupus erythematosus; RA, rheumatoid arthritis; SS, Sjögren's syndrome; IIM, idiopathic inflammatory myopathies; SSc, systemic sclerosis; CA, cancer
| Variables | All AIDs (n = 8120) | SLE (n = 3794) | RA (n = 1658) | SS (n = 1329) | IIM (n = 964) | SSc (n = 375) |
|---|---|---|---|---|---|---|
|
Median age at cohort entry (years, median [IQR]) |
43.8 (29.8-57.9) | 31.2 (21.1-41.4) | 57.8 (48.9-66.8) | 53.7 (43.6-63.7) | 48.3 (38.2-58.4) | 48.6 (38.8-58.4) |
|
Gender ratio (female/male) |
4.82 (6726/1394) | 6.66 (3299/495) | 3.02 (1246/412) | 9.55 (1203/126) | 2.31 (673/291) | 4.36 (305/70) |
|
Follow-up time (person-years) |
38726.55 | 19601.91 | 6787.29 | 6390.49 | 4144.29 | 1802.58 |
| Observed number of cancer (cases [%]) | 430 (100) | 90 (20.9) | 162 (37.7) | 84 (19.5) | 67 (15.6) | 27 (6.3) |
| Expected number of cancer (cases [%])† | 127.42 (100) | 34.92 (27.4) | 40.64 (31.9) | 29.18 (22.9) | 15.53 (12.2) | 7.16 (5.6) |
| SIR (95%CI) | 3.37 (3.06-3.71) | 2.58 (2.07-3.17) | 3.99 (3.40-4.65) | 2.88 (2.30-3.56) | 4.31 (3.34-5.48) | 3.77 (2.49-5.49) |
|
Incidence rate (per 100,000 person-years) |
1110.35 | 459.14 | 2386.82 | 1314.45 | 1616.68 | 1497.85 |
|
Age at cancer diagnosis (years, median [IQR]) |
57.5 (48.8-66.2) | 49.1 (39.8-58.5) | 61.5 (53.3-69.6) | 61.6 (51.8-71.5) | 59.7 (52.5-67.0) | 49.2 (43.1-55.3) |
|
Gender ratio of AID-Cancer patients (female/male) |
4.58 (353/77) | 21.50 (86/4) | 2.77 (119/43) | 15.80 (79/5) | 2.05 (45/22) | 8.00 (24/3) |
|
Time interval between AID and Cancer diagnosis (months, median [IQR]) |
79.8 (14.2-145.4) | 115.2 (62.4-166.8) | 121.2 (48.0-194.4) | 54.0 (4.8-103.2) | 7.2 (0-20.4) | 42.0 (3.6-80.4) |
- † Expected cancer was calculated by multiplying person-years and the cancer incidence of general Chinese population. The estimated cancer incidence rates were age, gender, and site-specific rates in 2006-2015, reported by the National Central Cancer Registry (NCCR) of China.
- Abbreviations: AID, autoimmune diseases; SLE, systemic lupus erythematosus; RA, rheumatoid arthritis; SS, Sjögren's syndrome; IIM, idiopathic inflammatory myopathies; SSc, systemic sclerosis. IQR: interquartile range; SIR, standardized incidence ratio; CI: confidence interval.
In the AID patients, the estimated SIR for all cancers was 3.37 (95% CI = 3.06-3.71), with the highest SIR observed in hematologic malignancies (SIR = 10.58; 95% CI = 7.93-13.84) followed by solid tumors (SIR = 3.09; 95% CI = 2.79-3.42). Among the five major AIDs, IIM had the highest SIR (4.31; 95% CI = 3.34-5.48), followed by RA (3.99; 95% CI = 3.40-4.65), SSc (3.77; 95% CI = 2.49-5.49), SS (2.88; 95% CI = 2.30-3.56), and SLE (2.58; 95% CI = 2.07-3.17).
3.2 Gender-, age-, geographic-, and site-specific SIRs
Gender discrepancies in cancer risk were observed, with the SIR of female patients (3.59; 95% CI = 3.23-3.99) being higher than that of male patients (2.77; 95% CI = 2.19-3.46). For female patients, the most common site of solid tumors was the vulva, followed by the urinary tract (bladder, ureter, and kidney), ovary, corpus uteri, and thyroid gland, with SIR values of 31.07 (95% CI = 10.09-72.51), 10.12 (95% CI = 5.39-17.31), 8.31 (95% CI = 5.43-12.17), 6.76 (95% CI = 4.28-10.14), and 6.60 (95% CI = 4.81-8.83), respectively (Figure 2A). The SIRs for complicated hematologic malignancies were also high, including non-Hodgkin's lymphoma (NHL; 20.82; 95% CI = 14.14-29.55), multiple myeloma (MM; 6.49; 95% CI = 1.34-18.98), and leukemia (3.27; 95% CI = 1.20-7.12). In male patients with AID, solid tumors commonly occurred in the gallbladder, kidney, and lung (SIR = 11.38, 95% CI = 3.10-29.14 for gallbladder; SIR = 8.88, 95% CI = 2.42-22.73 for kidney; and SIR = 4.02, 95% CI = 2.67-5.81 for lung). Similar to female patients, NHL showed an increased SIR in male patients(SIR = 20.19, 95% CI = 9.23-38.33).
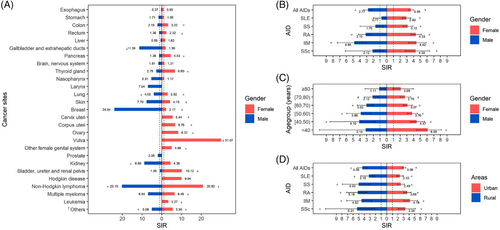
Gender- and age-specific standardized incidence ratios (SIRs) in AID patients and subgroups. (A) SIRs for cancer in each sites of female (red) and male (blue) patients. (B) SIRs for cancer in each type of AID in female (red) and male (blue) patients. (C) SIRs for cancer in different age groups (every 10 years) of female (red) and male (blue) patients. (D) SIRs for cancer in each type of AID patients living in urban (red) or rural areas (blue). *: SIRs significantly increased with 95% CI >1; † “Others” consists of cancers from eye, mouth, lip, parathyroid, thoracic neoplasms, bone, immunoproliferative diseases and connective tissue.
Abbreviations: SIR, standardized incidence ratio; AID, autoimmune disease; SLE, systemic lupus erythematosus; RA, rheumatoid arthritis; SS, Sjögren's syndrome; IIM, idiopathic inflammatory myopathies; SSc, systemic sclerosis
Among the five AID subgroups, all female patients had an increased cancer risk (2.90, 95% CI = 2.32-3.59 for SLE; 4.33, 95% CI = 3.59-5.18 for RA; 3.12, 95% CI = 2.47-3.88 for SS; 4.42, 95% CI = 3.22-5.91 for IIM; and 4.30, 95% CI = 2.75-6.39 for SSc). However, for male patients, increased SIR was only observed in those with IIM (4.84; 95% CI = 3.04-7.33) and RA (3.15; 95% CI = 2.29-4.22; Figure 2B).
In addition, younger patients (age <50 years) with AID had a higher cancer risk than older patients (age ≥50 years), with a gradually declining SIR observed from age >40 years to age ≥80 years (Figure 2C). Increased SIRs were observed in patients living in both urban and rural areas (Figure 2D).
3.3 Comparison of cancer distributions and site-specific SIRs among the five major AIDs
The detail information of all the analyzed patients with five major AIDs were summarized in Supplementary Table S3. Patients with SLE had significantly increased SIRs for developing hematologic malignancies (16.75, 95% CI = 7.66-31.80 for NHL; 5.14, 95% CI = 1.40-13.17 for leukemia) and solid tumors affecting the urinary bladder (19.96, 95% CI = 8.02-41.12), corpus uteri (5.38, 95% CI = 1.97-11.71), cervix uteri (4.80, 95% CI = 2.48-8.38), pancreas (5.20, 95% CI = 1.07-15.20), thyroid gland (4.19, 95% CI = 2.29-7.03), and colon (3.75, 95% CI = 1.22-8.74) (Figure 3A, Supplementary Table S4). Similar to SLE, patients with SS had high SIRs for developing NHL (24.88, 95% CI = 12.42-44.51) and solid tumors, including thyroid (8.41, 95% CI = 4.34-14.68), pancreas (6.86, 95% CI = 2.23-16.01), urinary tract (6.29, 95% CI = 1.30-18.39), cervical (4.18, 95% CI = 1.53-9.1), colon (4.06, 95% CI = 1.49-8.83), and lung (2.44, 95% CI = 1.26-4.26) cancers (Figure 3A, Supplementary Table S5). In patients with RA, a wide distribution of cancers was detected, with high SIRs for NHL (26.19, 95% CI = 14.97-42.53) and ovarian (13.66, 95% CI = 6.55-25.12), corpus uteri (12.40, 95% CI = 5.95-22.81), cervical (9.50, 95% CI = 4.91-16.59), thyroid (12.58, 95% CI = 7.19-20.43), kidney (9.60, 95% CI = 3.12-22.39), urinary tract (bladder, ureter, and renal pelvis; 4.29, 95% CI = 1.17-10.97), rectal (3.97, 95% CI = 1.71-7.82), breast (3.85, 95% CI = 2.15-6.35), and lung (3.70, 95% CI = 2.49-5.28) cancers (Figure 3A, Supplementary Table S6). The IIM group had a distinctive high risk of ovarian cancer (31.60, 95% CI = 15.77-56.53), followed by corpus uteri (9.89, 95% CI = 2.69-25.32), lung (5.94, 95% CI = 3.40-9.65), and breast (4.52, 95% CI = 2.07-8.59) cancers. IIM also showed a high SIR for developing NHL (12.66, 95% CI = 2.61-36.99) (Figure 3A, Supplementary Table S7). The SSc group showed the most increased SIRs for developing cervical uterine cancers (8.42, 95% CI = 1.74-24.61), followed by lung (6.69, 95% CI = 2.89-13.18), and breast (3.93, 95% CI = 1.07-10.07) cancers (Figure 3A, Supplementary Table S8).
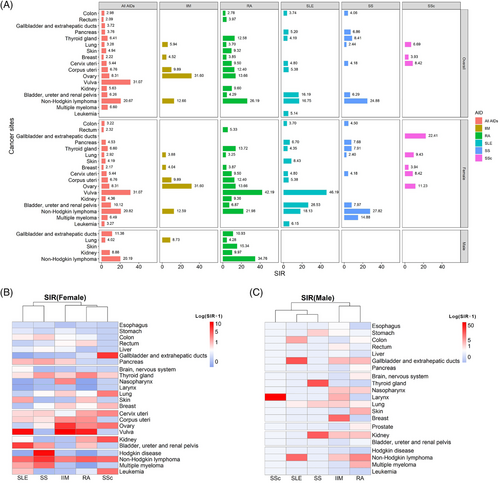
Site-specific standardized incidence ratios (SIRs) in AID patients and subgroups. (A) Cancer sites with significantly increased SIRs in all and five types of AIDs. (B) Heatmaps of SIRs for each cancer site in female patients, clustered by AIDs. (C) Heatmaps of SIRs for each cancer site in male patients, clustered by AIDs.
Abbreviations: SIR, standardized incidence ratio; AID, autoimmune disease; SLE, systemic lupus erythematosus; RA, rheumatoid arthritis; SS, Sjögren's syndrome; IIM, idiopathic inflammatory myopathies; SSc, systemic sclerosis
In both female and male patients, site-specific SIRs were further clustered by the five AID types and visualized using heatmaps. Patients with SLE and SS shared similarities, with an increased risk of developing hematological malignancies (Figure 3B and C).
3.4 Comparisons of the time interval between AID diagnosis and cancer occurrence among the five major AIDs
The time interval between AID diagnosis and cancer occurrence varied among the five AIDs. Approximately 30.2% (130/430) of the patients developed cancer in the first 3 years after the diagnosis of AID, with a declining curve of cancer in each triennium. Among the five AIDs, the time interval between IIM diagnosis and cancer occurrence was the shortest (7.2, IQR = 0-20.4 months). In the first triennium, 74.6% (50/67) of patients were diagnosed with cancer, of whom 55.2% (37/67) developed cancer in the first year. In the SSc and SS groups, the curve also decreased, with a large proportion of patients diagnosed with cancer during the first two trienniums (66.6% and 61.9%, respectively). The proportion of cancer patients in the SLE group was equal in each triennium. However, for patients with RA, the curve slightly elevated, showing that 29.0% (47/162) of the patients were diagnosed with cancer after a follow-up period of 15 years (Figure 4).
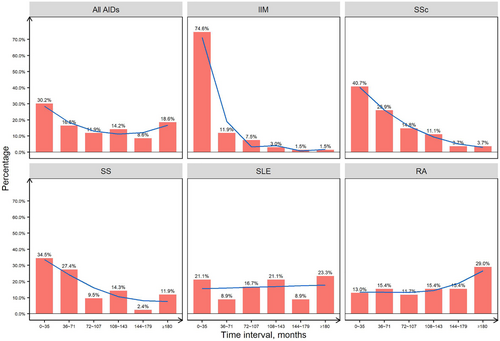
Time interval between the AID diagnosis and cancer diagnosis. Percentages of time interval between diagnosis of AID and cancer of AID-CA patients, with trend visualized by the smoothing line (blue line).
Abbreviations: AID, autoimmune disease; IIM, idiopathic inflammatory myopathies; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; SS, Sjögren's syndrome; SSc, systemic sclerosis, CA, cancer
4 DISCUSSION
A comprehensive depiction of the atlas of cancer distribution, incidence and time interval in AID patients will help in the prevention, early detection and proper management of cancer in AID paients. [26, 30] In this large-scale study in China, patients with the five major AIDs, including SLE, SS, RA, IIM and SSc, showed an increased risk of developing cancer, with a predominance in women and younger patients. The cancer incidence, predilection sites and cancer onset time varied greatly in each AID entity.
The risk of cancers increased in each AID group compared with that in the general population, which is consistent to the findings of previous studies conducted in Asia [31, 32]. The incidence of certain types of cancers, particularly hematologic malignancies (NHL and MM) and solid tumors affecting female urogenital organs, was particularly increased in Chinese AID patients compared with that in the general population. The increased risks of hematologic malignancies and cancer in the female genital organs have been reported in previous studies [12, 33-36]. Yet, the striking increase in the incidence of urinary tract tumor was possibly because cyclophosphamide was more vigorously applied in the treatment of Chinese AID patients compared with those in other regions worldwide [37].
It is intriguing to observe a female and age predilection for AID-complicated cancer in our study, which has scarcely been reported in previous studies. In addition, the female genital organs (vulva, ovary, and corpus uteri) were the most common sites of cancer affecting female patients with AID, followed by the urinary tract and the thyroid gland. This finding is in contrast with those observed in male patients; the latter had higher incidence of developing cancer in the gallbladder, kidney, and lung. This study also revealed that the cancer risk in younger patients (age <50 years) was generally higher than that in older patients (age ≥50 years). This may imply that AID is a stronger predisposing factor for cancer development in young patients; thus, more aggressive monitoring of cancer development in AID patients at a younger age is of utmost importance. As older age is a competitive risk factor for cancer, the impact of age on cancer risk in AID patients might become insignificant. Therefore, aggressive monitoring of cancer in patients with AID during their lifetime management is warranted.
This study confirmed that each AID had distinctive features related to cancer, suggesting that the mechanisms contributing to the increased cancer risk differ in each AID. Chronic inflammation, tumor neoantigen stimulating cross-active immune response, genetic alterations, and immunosuppressant use are potential factors that can cause cancer in patients with AIDs [6, 8, 13, 38-42]. IIM is associated with cancer. The overall cancer risk was highest in patients with IIM among the AID cohort. Consistent with previous findings, the risk of developing cancer was highest in the first year of IIM diagnosis [5, 34]; most cancers were diagnosed within 3 years after the onset of IIM. Therefore, vigorous cancer screening and monitoring are warranted at the onset of IIM, particularly at the urogenital system and lungs. Specifically, nasopharyngeal cancer was reported to be the most frequent malignancy associated with IIM in Southeast Asia and Southern China but not in North China, which was consistent with our findings [43, 44]. Anti-TIF1-γ antibody is a robust indicator of cancer development in patients with myositis, especially those with immunoglobulin G2 isotype [45]. Epitope spreading may contribute to cancer development in patients with IIM, with TIF1-γ overexpression detected in cancer tissues [6, 8].
Cancer remains a major concern in the long-term management of SLE. Our cohort revealed that SLE patients had a significantly increased risk of cancer, especially NHL, female genital organ cancer, and urinary tract cancer, which is in accordance with the findings of some previous studies [33, 46-50]. Viral infections, medication exposure, and aberrant immune responses may be implicated in the development of cancer in patients with SLE. Notably, the incidence of urinary bladder cancer in patients with SLE was much higher in our cohort (SIR 19.96, 95% CI 8.02-41.12) than in the reports of other countries worldwide (SIR 3.6, 95% CI 1.4-9.7) [51], possibly due to the extensive and vigorous application of cyclophosphamide in Chinese patients with SLE [12, 52]. SLE patients also have an increased risk of human papillomavirus-associated tumors (vulvar cancers and cervical dysplasia) and other potentially virus-induced cancers, such as lymphoma, which may be related to the impaired virus clearance in patients with SLE [36, 51, 53]. The overexpression of B-cell activating factor and proliferation-inducing ligand (APRIL) may also play a role in the increased risk of hematologic malignancies in patients with SLE because these cytokines affect the B-cell maturation [50].
SS showed a similarly increased cancer risk and distribution as SLE and a distinctively higher risk for NHL (SIR 24.88, 95% CI 12.42-44.51) in our cohort. Low-grade B-cell lymphomas (predominantly marginal zone histologic type) are prevalent in patients with SS-complicated NHL [54]. Chronic inflammation and B-cell aberrance in patients with SS may contribute to this risk. Other factors, including cryoglobulin-related markers, EULAR SS disease activity index, and lymphoid infiltration in the salivary gland, were predictive of lymphoma development in patients with SS [4, 54]. Lymphadenopathy, enlargement of the parotid glands, monoclonal immunoglobulin, and absence of hypergammaglobulinemia were previously reported as risk factors for malignancies in a Chinese cohort of SS [55].
In our RA cohort, the overall cancer risk increased (3.99, 95% CI 3.40-4.65), whereas other studies have reported a reduced cancer risk [10, 12]. Unlike in IIM, most cancers develop 9 years after the diagnosis of RA, suggesting that long-term monitoring of cancer is warranted in patients with RA. The potential carcinogenetic effects of disease-modifying anti-rheumatic drugs (DMARDs; e.g., methotrexate) and some biological agents (e.g., tumor necrosis factor α antagonists) in RA are noteworthy [13, 56].
In SSc, the risks of overall cancers and solid tumors increased, including cervical, lung, and breast cancers, which is consistent with the report of previous studies [7]. In addition, 40.7% of patients with SSc in our cohort developed cancer in the first three years, highlighting the need to closely monitor the risk of developing cancers in the early stage of SSc. Notably, the chronological order of AID and cancer was difficult to identify and may have influenced the calculation of SIRs. The SIRs of SSc patients in our study were higher than those reported in previous studies, which excluded cancer patients diagnosed within the first year of SSc diagnosis [7]. Similar to IIM, epitope spread may occur in patients with SSc who developed cancer. RNA polymerase III autoantibodies (POLR3A) indicated higher overall risks, whereas anti-centromere antibodies indicated reduced overall risk [57]. Mutations in POLR3A were identified as potential triggers for the co-occurrence of SSc and cancer [39].
Our study has several limitations. First, the patients in our cohort were recruited from a national tertiary referral center. Although our patients were from 31 provinces over mainland China, a geographic bias was inevitable. A nationwide database from multiple medical centers in China is expected to provide a more accurate estimate in the future. Second, this was a retrospective study; patients who were lost to follow-up were generally younger than the patients included, which may introduce a potential statistical bias and lead to overestimation of SIRs. Besides, the number of SSc patients was relatively small, and some types of cancers were too scarce for statistical analysis. Continuing follow-up is also required to validate the results. Third, this study only reported the epidemiological data of cancer patients with AIDs. Detailed analyses regarding clinical features (e.g., disease duration and activity), AID treatment, and comorbidities (e.g., diabetes, smoking, and alcoholism) were not conducted.
5 CONCLUSIONS
In conclusion, we reported an increased risk of cancers with a female predominance in Chinese patients with different types of AIDs, including SLE, SS, RA, IIM, and SSc. The site-specific cancer risk varied according to sex, age, and AID subtype. The trends of the time intervals from AID diagnosis to cancer occurrence also differed among the five AID groups. These findings provide a general description of the epidemiological association between AIDs and cancer in China. Considering the increased cancer risk in the long-term management of AID patients, increasing awareness and development of individualized cancer screening strategies for AID patients are warranted.
DECLARATIONS
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the ethics review boards of Peking Union Medical College Hospital (S-K1599). All patients involved has given written consents.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
COMPETING INTERESTS
The authors declare that they have no competing interests.
FUNDING
This study was supported by the grants from the National Natural Science Foundation of China (81801633, 81788101, and 81630044), Chinese Academy of Medical Science Innovation Fund for Medical Sciences (CIFMS 2020-I2M-C&T-B-011, 2021-I2M-1-017, 2021-I2M-1-047, 2021-I2M-1-040, and 2021-I2M-1-016), CSCO Pilot Oncology Research Fund (Y-2019AZMS-0452).
AUTHORS' CONTRIBUTIONS
Xuan Zhang and Huaxia Yang conceived and supervised the project. Ziyue Zhou, Huazhen Liu, Yiying Yang, and Jingya Zhou retrieved patients’ clinical information and conducted the telephone follow-up. Ziyue Zhou performed data curation, analysis and prepared data visualization. Huazhen Liu, Ziyue Zhou, and Yiying Yang, drafted the manuscript. Huaxia Yang, Xiaofeng Zeng reviewed and revised the manuscript. Lidan Zhao, Hua Chen, Yunyun Fei, Wen Zhang, Mengtao Li, Yan Zhao, Xiaofeng Zeng, Fengchun Zhang, Huaxia Yang and Xuan Zhang provided patient care and performed the clinical assessments. All authors have read and approved the manuscript.
ACKNOWLEDGEMENTS
We thank Dr. Yangzhong Zhou (Department of Internal Medicine, Chinese Academy of Medical Sciences and Peking Union Medical College Hospital, Beijing, China) for providing constructive suggestions regarding the study design and data presentation, as well as Dr. Zhuoran Yao (Department of Thoracic Cancer, Cancer Center, Clinical Cell Therapy Laboratory, West China Hospital, West China School of Clinical Medicine Sichuan University, Sichuan, China) and Dr. Xiaoxiang Zhou (Department of Thoracic Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China) for providing practical advice. We would also thank Xinyi Chen, Ruichen Gao, Qun Gu, Peida Han, Yixuan Huang, Siqi Jiang, Xindi Ke, Shubin Lei, Bo Li, Yaqi Li, Tingting Liu, Xingtong Peng, Jiangming Qu, Yixin Sun, Lu Wang, Zhaojue Wang, Ming Wu, Yu Xia, Biyun Zhang, and Rongjun Zhou (Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China) for their engagement in the telephone follow-up of patients.