JUPITER-02 trial: advancing survival for recurrent metastatic nasopharyngeal carcinoma and next steps
List of abbreviations
-
- NPC
-
- nasopharyngeal carcinoma
-
- RT
-
- radiotherapy
-
- GP
-
- gemcitabine-cisplatin
-
- SOC
-
- standard-of-care
-
- PF
-
- cisplatin and 5-fluorouracil
-
- R/M-NPC
-
- recurrent/metastatic nasopharyngeal carcinoma
-
- PFS
-
- progression-free survival
-
- OS
-
- overall survival
-
- SYSUCC
-
- Sun Yat-Sen University Cancer Centre
-
- EBV
-
- Epstein-Barr virus
-
- OR
-
- overall response
-
- DoR
-
- duration of response
-
- HR
-
- Hazard ratio
-
- CI
-
- confidence intervals
-
- AE
-
- adverse event
-
- PD-L1
-
- programmed death ligand-1
Nasopharyngeal carcinoma (NPC) is a disease with unique epidemiology. It is endemic in Southern China, Southeast Asia, and North Africa, and typically affects males more than females in the 30-60 years age group [1]. Globally, 133,354 NPC cases were diagnosed in 2020 with 80,008 deaths in the same year [2]. While the survival rates of early-stage NPC after radiotherapy (RT) are high, disease recurrences are common in locoregionally-advanced NPC, with the predominant pattern of failure being distant metastasis [3]. In this clinical scenario, palliative chemotherapy is the primary treatment modality, and the doublet regimen of gemcitabine-cisplatin (GP) was the current treatment of choice until this year. This standard-of-care (SOC) was established on the backbone of results of a seminal randomized controlled phase III clinical trial of GP versus the historical regimen of cisplatin and 5-fluorouracil (PF) that was conducted in China. On a laudable note, this randomized comparison was the first ever phase III clinical trial to be conducted in recurrent/metastatic NPC (R/M-NPC). The results of the trial were groundbreaking. The investigators reported that simply replacing 5-fluorouracil with gemcitabine prolonged progression-free survival (PFS) from 5.6 months to 7.0 months and overall survival (OS) from 18.6 months to 22.1 months [4].
Riding on the success of this trial, investigators from the Sun Yat-Sen University Cancer Centre (SYSUCC) and their international colleagues from Taiwan and Singapore conceptualized a subsequent randomized controlled phase III trial in R/M-NPC, recognizing the need for better combinatorial regimens, since only ∼20% of patients remained progression-free at 1-year post-GP. To this end, they leveraged on the fact that endemic NPC is strongly associated with Epstein-Barr virus (EBV) infection, and this tumor is characterized by a high peritumoral immune infiltrate that harbors an exhausted phenotype [5]. Thus, they rationalized that immune checkpoint blockade therapy may be synergistic with GP, with early-phase smaller-scale studies supporting this hypothesis [6, 7]. The JUPITER-02 study was initiated and recruited 289 patients with R/M-NPC disease, in a double-blinded manner, to receive either toripalimab or placebo together with GP during the induction phase, followed by maintenance with toripalimab or placebo until disease progression or a maximum of two years of therapy, whichever is earlier [8]. Crossover was not permitted on the trial. The primary endpoint at the prespecified interim analysis was PFS, while OS, overall response (OR) and duration of response (DoR) and safety consisted of the secondary endpoints. Toripalimab significantly improved PFS compared to the placebo arm (hazard ratio [HR] = 0.52, 95% confidence intervals [CI] = 0.36-0.74 based on central review; HR = 0.41, 95% CI = 0.28-0.59 based on investigator-assessment). At 1 year, 49.4% of patients in the experimental arm remained progression-free compared to 27.9% in the control arm, and this difference was even more stark when assessed by investigators on the trial (∆39.5%). Interestingly, OR was not substantially different between the arms (∼10%), but DoR was significantly longer with toripalimab (10.8 months vs. 5.7 months), suggesting that toripalimab may be exerting its effects by modulating the immune response against occult metastatic tumor clones. The OS results are not yet mature, but preliminary interpretation suggests that the PFS benefit may be translated to an OS benefit (stratified HR = 0.60, 95% CI = 0.36-0.99). Finally, severe adverse events (AEs) leading to discontinuation of therapy and fatal reactions were comparable between both arms (7.5% vs. 4.9% and 2.7% vs. 2.8%, respectively), but immune-related AEs (39.7% vs. 18.9%) and grade ≥3 infusion reactions (7.5% vs. 0.7%) were expectedly more common among patients who received toripalimab. In summary, these results mark another major milestone in R/M-NPC, as patients now have a better, potentially life-prolonging treatment in a mere span of 5 years from the preceding GP versus PF trial.
The results of JUPITER-02 are supported by two other randomized controlled trials (CAPTAIN-1st and RATIONALE-309 [NCT03924986, ClinicalTrials.gov]) investigating the roles of immune checkpoint blockade therapy in combination with GP in the treatment of R/M-NPC. Between them, only the investigators of CAPTAIN-1st had formally reported their results with camrelizumab in the same time period as JUPITER-02 (Supplementary Table S1) [9, 10], while the results of RATIONALE-309 (using tislelizumab as the experimental agent) were only preliminarily announced by BeiGene (Beijing, China) via a press release. Likewise to JUPITER-02, CAPTAIN-1st showed that PFS was significantly longer in the camrelizumab than placebo arm (median of 10.8 months vs. 6.9 months, HR = 0.51, 95% CI = 0.37-0.69); OS results were immature at the time of reporting [10]. Both cohorts appeared comparable for known prognostic clinical parameters (recurrent vs. de novo, pre-treatment EBV DNA titer, and sites of metastases such as lung and liver involvement). Consistent with the notion that immune checkpoint inhibitors do harbor subtle differences in toxicities, the investigators observed significantly higher rates of serious AEs in the camrelizumab arm (44%) compared to the placebo arm (37%). There were also five (4%) treatment-related deaths among those who received camrelizumab [9, 10]. Another key point to make is that programmed death ligand-1 (PD-L1) status was not assessed in the CAPTAIN-1st trial. Interestingly, in the JUPITER-02 study, toripalimab showed efficacy independent of PD-L1 status (HR: 0.35 for PD-L1 negative vs. 0.59 for PD-L1 positive), even though PD-L1 negative (<1% positive staining) tumors comprised a minority (17.1%) of the cohort. This would suggest that other pathways and molecular characteristics (i.e. tumor mutational burden status) are involved in driving the positive effects of inhibiting the PD-1/PD-L1 axis in R/M-NPC [7].
The recent publication of these findings now begets the question of whether these results impact real-world clinical practice, as well as how we can improve upon the results from from JUPITER-02 and CAPTAIN-1ST (Figure 1). Firstly, it is important to acknowledge that mature OS is still pending from these trials, so one will be curious to see how these impressive results for PFS translate to OS outcomes, especially when the benefits of immune checkpoint blockade treatment are more substantial for OS than PFS, as observed in other cancer types [7, 11, 12]. Next, patients with moderate or severe hepatic or renal impairment were excluded from the trials, which begs the question if either toripalimab or camrelizumab can be safely used in this patient population when it comes to real-world practice. Thirdly, toripalimab and camrelizumab are currently not available to patients outside of China, unlike pembrolizumab and nivolumab that have broader market access. Can the data of JUPITER-02 and CAPTAIN-1st be extrapolated to the other checkpoint inhibitors? Fourthly, these life-prolonging therapies are not necessarily cost-effective. We thus ask the question if their use can be limited to the maintenance phase of treatment, rather than upfront concurrently with GP, without compromising on the efficacy. Judging by the separation of the PFS curves for JUPITER-02 and CAPTAIN-1st, it is plausible that the anti-tumor effects of toripalimab and camrelizumab are most potent post-cytotoxic chemotherapy. On this note, we also raise the possibility regarding the efficacy and safety of combining anti-PD-L1 antibody with consolidation radiotherapy in patients with de novo metastatic NPC, given the positive trial by You et al. [13] supporting the addition of consolidation radiotherapy in chemo-responsive NPC patients. This sequential strategy may yield further survival improvement in R/M-NPC patients, notwithstanding the promise of combinatorial immunotherapeutic regimens that simultaneously target multiple pathways in the immunological ecosystem of NPC [14].
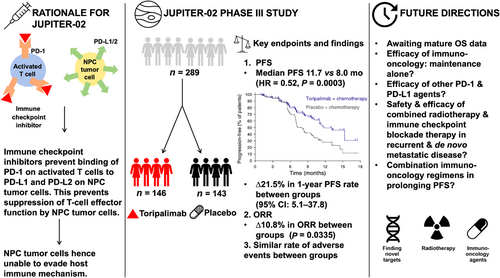
It was not too distant when five years ago it took three years for a village to complete recruitment for a randomized controlled phase III trial in R/M-NPC, and another year to report the results. Nonetheless, the success of the GP versus PF trial had catalyzed the field to conduct several large-scale trials in R/M-NPC. Impressively, both JUPITER-02 and CAPTAIN-1st were completed within a period of less than two years, and the early results have presented a new standard-of-care in this aggressive subgroup of patients. The investigators from both trials ought to be congratulated, but there is still ample room to improve the efficacy and safety profiles of these checkpoint inhibitors. Sequential combinatorial strategies may be a way forward in R/M-NPC. We call on the NPC medical community to collectively push on to advocate for more of such trials, without which, we risk losing the momentum of these early successes and limit progress in advancing survival for these patients.
DECLARATIONS
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
COMPETING INTERESTS
The authors declare no direct conflict of interest for this work. M.L.K.C. reports personal fees from Astellas, Janssen, Bayer, Pfizer, MSD, Varian, Telix Pharmaceuticals, personal fees and non-financial support from AstraZeneca, personal fees and grants from Ferring, non-financial support from Decipher Biosciences, non-financial support from MedLever, and consults for immunoSCAPE Inc., outside the submitted work.
FUNDING
M.L.K.C. is supported by the National Medical Research Council Singapore Clinician Scientist Award (NMRC/CSA-INV/0027/2018, CSAINV20nov-0021), the Duke-NUS Oncology Academic Program Goh Foundation Proton Research Programme, NCCS Cancer Fund, and the Kua Hong Pak Head and Neck Cancer Research Programme.
AUTHORS' CONTRIBUTIONS
Study conception: M.L.K.C. Collection and assembly of data: L.L.Y.T. and M.L.K.C. Administrative and funding support: M.L.K.C. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
The authors thank members of the Chua's lab for their critical comments.




