Paclitaxel liposome for injection (Lipusu) plus cisplatin versus gemcitabine plus cisplatin in the first-line treatment of locally advanced or metastatic lung squamous cell carcinoma: A multicenter, randomized, open-label, parallel controlled clinical study
Abstract
Background
Lipusu is the first commercialized liposomal formulation of paclitaxel and has demonstrated promising efficacy against locally advanced lung squamous cell carcinoma (LSCC) in a small-scale study. Here, we conducted a multicenter, randomized, phase 3 study to compare the efficacy and safety of cisplatin plus Lipusu (LP) versus cisplatin plus gemcitabine (GP) as first-line treatment in locally advanced or metastatic LSCC.
Methods
Patients enrolled were aged between 18 to 75 years, had locally advanced (clinical stage IIIB, ineligible for concurrent chemoradiation or surgery) or metastatic (Stage IV) LSCC, had no previous systemic chemotherapy and at least one measurable lesion as per the Response Evaluation Criteria in Solid Tumors (version 1.1) before administration of the trial drug. The primary endpoint was progression-free survival (PFS). The secondary endpoints included objective response rate (ORR), disease control rate (DCR), overall survival (OS), and safety profiles. To explore the possible predictive value of plasma cytokines for LP treatment, plasma samples were collected from the LP group at baseline and first efficacy evaluation time and were then subjected to analysis by 45-Plex ProcartaPlex Panel 1 to detect the presence of 45 cytokines using the Luminex xMAP technology. The correlation between treatment outcomes and dynamic changes in the levels of cytokines were evaluated in preliminary analyses.
Results
The median duration of follow-up was 15.4 months. 237 patients in the LP group and 253 patients in the GP group were included in the per protocol set (PPS). In the PPS, the median PFS was 5.2 months versus 5.5 months in the LP and GP group (hazard ratio [HR]: 1.03, P = 0.742) respectively. The median OS was 14.6 months versus 12.5 months in the LP and GP group (HR: 0.83, P = 0.215). The ORR (41.8% versus 45.9%, P = 0.412) and DCR (90.3% versus 88.1%, P = 0.443) were also similar between the LP and GP group. A significantly lower proportion of patients in the LP group experienced adverse events (AEs) leading to treatment interruptions (10.9% versus 26.4%, P < 0.001) or treatment termination (14.3% versus 23.1%, P = 0.011). The analysis of cytokine levels in the LP group showed that low baseline levels of 27 cytokines were associated with an increased ORR, and 15 cytokines were associated with improved PFS, with 14 cytokines, including TNF-α, IFN-γ, IL-6, and IL-8, demonstrating an overlapping trend.
Conclusion
The LP regimen demonstrated similar PFS, OS, ORR and DCR as the GP regimen for patients with locally advanced or metastatic LSCC but had more favorable toxicity profiles. The study also identified a spectrum of different cytokines that could be potentially associated with the clinical benefit in patients who received the LP regimen.
Abbreviations
-
- AE
-
- adverse events
-
- CI
-
- confidence interval
-
- CR
-
- complete response
-
- DCR
-
- disease control rate
-
- ECOG PS
-
- Eastern Cooperative Oncology Group performance status
-
- FAS
-
- full analysis set
-
- HR
-
- hazard ratio
-
- IASLC
-
- International Association for the Study of Lung Cancer
-
- ICI
-
- immune checkpoint inhibitor
-
- ITT
-
- intention-to-treat
-
- LP
-
- cisplatin plus Lipusu GP, cisplatin plus gemcitabine
-
- LSCC
-
- lung squamous cell carcinoma
-
- Nab-Paclitaxel
-
- Nanoparticle albumin-bound (nab-) paclitaxel
-
- NSCLC
-
- non-small cell lung cancer
-
- ORR
-
- objective response rate
-
- OS
-
- overall survival
-
- PD-1
-
- programmed cell death 1
-
- PD-L1
-
- /PD ligand-1
-
- PFS
-
- progression-free survival
-
- PPS
-
- per protocol set.
-
- PR
-
- partial response
-
- RECIST
-
- Response Evaluation Criteria in Solid Tumors
-
- SAE
-
- severe adverse events
-
- SAS
-
- Statistical Analysis System software
-
- SD
-
- stable disease
1 INTRODUCTION
Lung squamous cell carcinoma (LSCC), a major histologic subtype of non-small cell lung cancer (NSCLC), accounts for approximately 20%-30% of lung cancer cases [1]. Despite remarkable advances in the treatment of non-squamous NSCLC with the advent of promising targeted therapies against EGFR, ALK, and ROS1, treatment options remain rather limited for LSCC patients because of their distinct clinicopathological traits from non-squamous NSCLC patients as well as the rarity of the presence of EGFR mutations [2, 3] and ALK rearrangements [4] and there is currently no approved targeted therapy for patients with LSCC. Developing novel therapeutic strategy to improve the survival of patients with metastatic LSCC remains an unmet medical need.
Notably, the therapeutic landscape for LSCC has changed significantly over recent years with the introduction of immune checkpoint inhibitors (ICIs) that can effectively target programmed cell death 1 (PD-1)/PD ligand-1 (PD-L1) pathway. ICIs, either as monotherapy or in combination with chemotherapy, have also been advocated as the standard of care for patients with metastatic LSCC in first-line treatment. However, for patients who may not be suitable for ICI treatment, histology-oriented chemotherapy still plays an important role in the management of metastatic LSCC. Currently, for patients with metastatic LSCC, first-line chemotherapy therapeutic regimens include cisplatin or carboplatin combined with cytotoxic agents, including gemcitabine, paclitaxel, and docetaxel [5, 6].
Paclitaxel plus platinum is one of the most commonly used regimens for patients diagnosed with LSCC. However, solvent-based paclitaxel has been associated with severe allergies [7] and toxic reaction [8] as it is highly lipophilic as well as hydrophobic and is often dissolved in the mixed solvents containing Cremophor EL (polyoxyethylated castor oil). Paclitaxel disposition in the body has been found to vary considerably depending on the formulations used. Nanoparticle albumin-bound (nab-) paclitaxel is an albumin-bound, solvent-free, nanoparticle form of paclitaxel that displays some advantages over solvent-paclitaxel, including those of higher delivery doses of paclitaxel in vivo and more favorable toxicities. However, nab-paclitaxel needs to be administrated weekly and is expensive in China. Interestingly, in vivo studies have also demonstrated that liposome-paclitaxel could significantly increase the maximum tolerated dose by 2-7 times [9-12] and could also reduce toxicities without compromising its anti-tumor efficacy [13], compared with the solvent-paclitaxel formulation.
Lipusu, the first commercialized liposomal formulation of paclitaxel (Nanjing Luye Pharmaceutical Co., Ltd.), has been shown to exhibit first-order elimination in a three-compartment model in NSCLC patients. In patients with metastatic gastric cancer, Lipusu has been as effective as paclitaxel and displayed significantly lesser adverse events (AEs), such as nausea and vomiting (23% vs. 50%), dyspnea (0% vs. 18%), peripheral neuritis (3% vs. 32%) and more [14]. A small-scale clinical study of 38 patients with locally advanced LSCC showed that liposome-paclitaxel and carboplatin with concurrent radiotherapy achieved an objective response rate (ORR) of 68.4% (26/38) with manageable toxicities [15]. However, currently, no data is available on the potential efficacy and safety of Lipusu in locally advanced or metastatic LSCC as first-line treatment in a prospective randomized setting.
Cytokines are soluble proteins that can effectively mediate cell-to-cell communication [16]. Considering the ability of the immune system to recognize and attack cancer cells, there has been considerable interest in characterizing the predictive value of cytokines for cancer therapy. A number of previous clinical studies have suggested that simultaneous immunostimulation and immunosuppression could occur in patients with advanced-stage cancer and these processes have been associated with increased concentrations of the cytokines such as tumor necrosis factor α (TNF-α), macrophage migration inhibitory factor (MIF), transforming growth factor β (TGF-β), interleukin [IL]-18 (IL-18) and IL-8 [17]. Additionally, accumulating evidences have also indicated that circulating cytokines could serve as predictive biomarkers for the efficacy of systemic therapy, including chemotherapy, targeted therapy and immunotherapy in different cancers [18-22].
In this study, we conducted a multicenter, randomized, open-label, parallel controlled trial to evaluate the potential efficacy and safety of cisplatin plus Lipusu (LP) versus cisplatin plus gemcitabine (GP), another standard regimen of chemotherapy for LSCC that is commonly used in China, as the first-line treatment for patients with locally advanced or metastatic LSCC. To explore the predictive value of plasma cytokines for LP treatment in locally advanced or metastatic advanced LSCC, we also evaluated the correlation between the baseline and dynamic changes of cytokines and treatment outcomes in the LP group for exploratory analyses.
2 PATIENTS AND METHODS
2.1 Study design and participants
The trial was conducted at 35 different hospitals across China between December 2016 and December 2019. Eligible patients were aged between 18 and 75 years with histologically or cytologically confirmed stage IIIB-IV LSCC. The approaches to obtain histological specimens included computer tomography-guided percutaneous lung puncture biopsy and transbronchial lung biopsy. For patients with cytological specimens, p40 or p63 by immunohistochemistry (IHC) were used to confirm the diagnosis and was a standard way of diagnosis at all the participating institutions. LSCC was staged according to the 2009 International Association for the Study of Lung Cancer (IASLC) lung cancer staging project for tumor-node-metastasis (TNM) classification. Patients who had received no prior chemotherapy, biotherapy, immunotherapy, or who experienced recurrence and metastasis more than 6 months after the last adjuvant chemotherapy (excluding gemcitabine and paclitaxel), with an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1 were eligible for enrollment. All patients had at least one measurable lesion according to version 1.1 of the Response Evaluation Criteria in Solid Tumors (RECIST 1.1) [23]. The details of the study design and patient eligibility criteria are available in https://clinicaltrials.gov (NCT02996214).
The whole blood samples of patients enrolled in this clinical trial were collected at different time points, including baseline when patients were randomized, the time when patients were evaluated for efficacy, and the time when the disease has progressed. The whole blood samples collected were centrifuged for two hours to isolate plasma for preliminary biomarker analysis.
This trial was conducted in accordance with the Declaration of Helsinki. The independent ethics committee or institutional review board of each participating study center approved the protocol and all amendments. All the participants provided written informed consent before enrollment in this trial.
2.2 Randomization and treatment
Block randomization was used wherein a computer-generated randomization sequence in blocks was prepared using the Statistical Analysis System software version 9.4 (SAS 9.4) PROC PLAN. The participants were randomized in a 1:1 ratio to receive paclitaxel liposome for injection (Lipusu®,175 mg/m2, day 1, results from a phase II study recommended a Lipusu of 175 mg/m2 triweekly as a component of the combination chemotherapy [24]) plus cisplatin (75 mg/m2, day1) (LP group), or gemcitabine (1000 mg/m2, day 1 and 8) plus cisplatin (75 mg/m2, day 1) (GP group) for 4-6 cycles at the discretion of investigators, until unacceptable toxicities, disease progression, or withdrawal of consent. Dose adjustments were allowed as per label for resolution of the adverse events. Specifically, for Lipusu, dose reduction was allowed if grade 4 hematologic adverse events or ≥ grade 3 non-hematologic toxicity occurred (first reduction to 155 mg/m2 triweekly, second reduction to 135 mg/m2 triweekly, and only two-dose reductions were allowed during treatment course). Randomization was stratified by bodyweight reduction (≥5% vs. <5% within 6 months), age (≥ 65 vs. < 65 years), and TNM stage (IIIB vs. IV).
2.3 Endpoints and assessments
The primary endpoint was progression-free survival (PFS), calculated from the date of randomization to the date of disease progression or death from any cause. The secondary endpoints included ORR, which was the proportion of patients who achieved complete response (CR) or partial response (PR) according to RECIST 1.1; disease control rate (DCR), which was the proportion of patients who achieved stable disease (SD), CR or PR, and; overall survival (OS), which was calculated from the date of randomization to the date of death from any cause.
Tumor assessment was performed every 2 cycles (42 ± 3 days) following the RECIST 1.1 criteria [23]. The response confirmation occurred 4 weeks or more after an initial measurement and every other cycle thereafter. After disease progression, the follow-up was conducted every 90 days by telephone until imaging examination proved progressive disease (PD) or death or lost during follow-up. AEs were followed up to within 21 ± 3 days after the last administration of the study drugs.
2.4 Safety
The safety was assessed weekly based mainly on the occurrence, frequency, and severity of AEs by using the National Cancer Institute-Common Terminology Criteria for Adverse Events (NCI CTCAE) version 4.0 and coded to a preferred term using the Medical Dictionary for Regulatory Activities (MedDRA). The safety events included AEs and severe AEs (SAEs). For all AEs, wherever necessary, patients were withdrawn from the study.
2.5 Detection of the levels of cytokines
Plasma samples from 92 LSCC patients in the LP group were obtained at baseline, the first efficacy evaluation was carried out after administration of the two cycles of LP regimen and disease progression. To isolate plasma, ethylenediaminetetraacetic acid (EDTA)-anticoagulated whole blood samples were centrifuged (1000 × g, 15 min). A total of 45 cytokines were simultaneously measured in plasma samples using the Cytokine/Chemokine/Growth Factor 45-Plex Human ProcartaPlex Panel (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The concentration of the cytokines used was the expression level of the cytokines. To define potential “cytokine signatures” in the plasma, the 45 peripheral immune factors were classified into different subgroups according to their biological characterization (e.g., growth factors, chemokines, interferons, interleukins, and colony stimulatory factors). After log2 transformation of the concentration value, a signature score was calculated by averaging the various included cytokines. Univariate Cox regression analysis was used to screen for potential signatures associated with ORR and PFS. A detailed list of all detected cytokines is provided in Supplementary Table S1.
2.6 Statistical analyses
The sample size was based on a reported PFS of 5 months for locally advanced or metastatic LSCC patients receiving first-line gemcitabine plus cisplatin, and the expected PFS of patients receiving paclitaxel liposome plus cisplatin was 6.5 months. For the enrolment period of 18 months and study period of 36 months, to obtain a statistically significant result of mid-term analysis, a total of 466 PFS events were needed. By assuming two-sided α = 0.05, β = 20% and a dropout rate of at least 20%, the total sample size was calculated to be 536.
Statistical analyses were pre-specified and followed the intention-to-treat (ITT) principle which implied that all participants randomized should be included in the statistical analysis and analyzed according to the group they were originally assigned, regardless of the treatment regimen administered to them. The full analysis set (FAS) included all patients who received at least one dose of the study medication, had a baseline evaluation and at least one post-baseline evaluation. The per protocol set (PPS) included patients who met the eligibility criteria and did not show any major violation of the study protocol, completed the treatment, underwent efficacy evaluation and showed good compliance. Patients who did not receive full evaluation for efficacy were censored. A mid-term and a final analysis were conducted using the O'Brien-Fleming alpha spending function approach. The significance levels assigned to the mid-term and final analysis were α1 = 0.005 and α2 = 0.048, respectively.
For the cytokine study, the Wilcoxon rank-sum test and Wilcoxon signed-rank test were performed to determine the possible differences between two independent or paired subgroups of cytokines, respectively. Univariate Cox regression analysis was used to screen the cytokine signatures associated with ORR and PFS. PFS analysis was performed using the Kaplan-Meier method and log-rank test.
The safety set included all patients who received at least one dose of the study drug, had at least one follow-up safety assessment and were analyzed mainly using descriptive statistics.
All statistical analyses were conducted using the SAS software package, version 9.4 (SAS Institute Inc., Cary, NC), GraphPad Prism 8.0 and the SPSS statistical software, version 24.0 (SPSS Inc., Chicago, IL, USA). All the tests were two-tailed with a level of significance set at α = 0.05 except for testing the primary endpoint.
3 RESULTS
3.1 Patient demographic and baseline characteristics
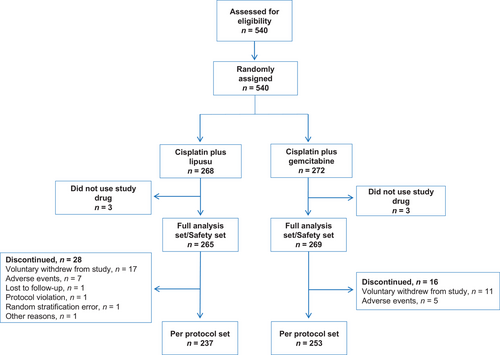
The study flowchart has been shown in Figure 1. Five hundred forty patients underwent screening for eligibility and were randomized to receive LP regimen (n = 268) or GP regimen (n = 272). Three patients from each group did not receive the study medication and were excluded from the study. The FAS included 265 patients in the LP group and 269 patients in the GP group. One hundred seventy-seven (33.2%) patients had stage IIIb LSCC and 357 (66.9%) patients had stage IV LSCC. The demographic and baseline variables were well-balanced between the two different treatment groups (Table 1).
| Variables | All (n = 534) | LP (n = 265) | GP (n = 269) | P value |
|---|---|---|---|---|
| Mean ± SD age, years | 61.3 ± 7.3 | 61.2 ± 7.3 | 61.5 ± 7.3 | 0.623 |
|
Male gender, n (%) Female gender, n (%) |
497 (93.1) 37 (6.9) |
245 (92.5) 20 (7.6) |
252 (93.7) 17 (6.3) |
0.577 |
| Mean ± SD, body weight, kg | 62.7 ± 9.7 | 62.5 ± 9.3 | 62.8 ± 10.1 | 0.710 |
|---|---|---|---|---|
| Weight loss*, n (%) | 0.391 | |||
| < 5% | 358 (67.0) | 173 (65.3) | 185 (68.8) | |
| ≥ 5% | 176 (33.0) | 92 (34.7) | 84 (31.2) |
| ECOG PS, n (%) | 0.847 | |||
|---|---|---|---|---|
| 0 | 87 (16.3) | 44 (16.6) | 43 (16.0) | |
| 1 | 447 (83.7) | 221 (83.4) | 226 (84.0) | |
| Clinical stage, n (%) | 0.691 | |||
| IIIb | 177 (33.2) | 90 (34.0) | 87 (32.3) | |
| IV | 357 (66.9) | 175 (66.0) | 182 (67.7) |
| Smoking status, n (%) | 0.078 | |||
|---|---|---|---|---|
| Never | 67 (12.6) | 40 (15.1) | 27 (10.0) | |
| Former/current | 467 (87.5) | 225 (84.9) | 242 (90.0) | |
| Family history of tumor, n (%) | ||||
| Yes | 81 (15.2) | 39 (14.7) | 42 (15.6) | 0.809 |
- Abbreviation: ECOG PS, Eastern Cooperative Oncology Group performance status; SD, standard deviation;
- * Weight loss in the preceding 6 months.
3.2 Treatment characteristics
Patients in the LP and GP group received a mean (± standard deviation) of 4.4 ± 1.8 and 4.5 ± 1.7 cycles of chemotherapy, respectively. 73.2% of patients in the LP group and 74.0% of patients in the GP group completed four or more cycles of chemotherapy. Significant dose reductions were required in 33.2% of patients in the LP group and 27.1% of patients in the GP group. Furthermore, 11.3% of patients in the LP group and 26.4% of patients in the GP group experienced treatment interruptions, respectively (Table 2).
| Variables | LP, n = 265, n (%) | GP, n = 269, n (%) |
|---|---|---|
| No. of treatment cycles, n (%) | ||
| 1 | 26 (9.8) | 18 (6.7) |
| 2 | 31 (11.7) | 34 (12.6) |
| 3 | 14 (5.3) | 18 (6.7) |
| 4 | 53 (20.0) | 53 (19.7) |
| 5 | 19 (7.2) | 27 (10.0) |
| ≥ 6 | 122 (46.0) | 119 (44.2) |
| Mean ± SD | 4.4 ± 1.8 | 4.5 ± 1.7 |
| Actual total dosage (mg) | ||
| Mean ± SD | 1276.7 ± 527.6 | 14407.9 ± 5830.9 |
| Median (Q1-Q3) | 1380.0 (900.0-1720.0) | 15360.0 (10240.0-19200.0) |
| Dosage range | 250-2202 | 1480-24000 |
| Dose interruptions, n (%) | ||
| No. of treatment cycles, n (%) | 235 (88.7) | 198 (73.6) |
| 1 | 25 (9.4) | 46 (17.1) |
| 2 | 4 (1.5) | 16 (6.00) |
| > 2 | 1 (0.4) | 9 (3.4) |
| Dose modification, n (%) | ||
| No reduction | 177 (66.8) | 196 (72.9) |
| Reduction (Lipusu and gemcitabine) | 88 (33.2) | 73 (27.1) |
| 175 mg/m2→155 mg/m2/ and 1000 mg/m2→800 mg/m2 | 87 (98.9) | 73 (100.0) |
| 155 mg/m2→135 mg/m2/ and 800 mg/m2→600 mg/m2 | 19 (21.6) | 5 (6.9) |
| 175 mg/m2→135 mg/m2/ and 1000 mg/m2→600 mg/m2 | 1 (1.1) | 0 (0.0) |
- Abbreviation: SD, standard deviation; LP, cisplatin and lipusu; GP, cisplatin and gemcitabine.
3.3 Efficacy results
The last date of follow-up was on December 10, 2019, and the median duration of follow-up was 15.4 months (range: 0.2 to 34.5 months). One patient was lost during follow-up. 237 patients in the LP group and 253 patients in the GP group were included in the PPS. In the PPS, the median PFS was 5.2 months (95% confidence interval [CI], 4.7-5.6) in the LP group and 5.5 months (95% CI, 5.1-5.8) in the GP group (HR: 1.03, P = 0.742) (Figure 2A). The median OS in the LP group was 14.6 months (95% CI 12.4-15.8) and was 12.5 months in the GP group (95% CI 10.8-14.6) (HR: 0.86, P = 0.215) (Figure 2B). There were no significant differences between the two groups in terms of PFS (P = 0.742) and OS (P = 0.215) in the PPS. Across most subgroups that were analyzed, the LP group exhibited a similar PFS compared with the GP group (Figure 2C).
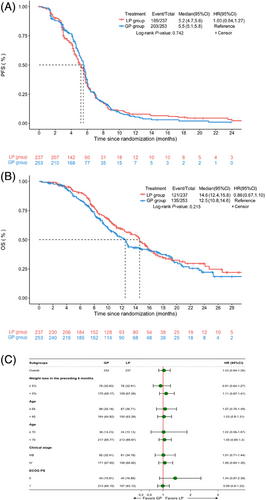
The Kaplan-Meir curves of progression-free survival (A) and overall survival (B) of the full analysis set stratified by treatment with LP and GP. (C) Forest plots for effects related to the bodyweight reduction (≥ 5% vs. < 5% within 6 months), age (≥ 65 vs. < 65 years; ≥ 70 vs. < 70 years), and TNM stage (IIIB vs. IV), and ECOG PS (0 versus 1) on progression-free survival. Abbreviations: LP, cisplatin plus Lipusu; GP, cisplatin plus gemcitabine; ECOG PS, Eastern Cooperative Oncology Group performance status; ys, years; HR, hazard ratio; CI, confidence interval
In the PPS, there were no significant differences between the LP group and the GP group regarding ORR (LP: 41.8% vs. GP: 45.9%, P = 0.412), and DCR (LP: 90.3% vs. GP: 88.1%, P = 0.443) (Table 3). The results of PPS were found to be generally consistent with those of FAS (Supplementary Table S2).
| Response | LP, n = 237, n (%) | GP, n = 253, n (%) | P value |
|---|---|---|---|
| CR | 1 (0.4) | 0 (0.0) | |
| PR | 98 (41.4) | 116 (45.9) | |
| SD | 115 (48.5) | 107 (42.3) | |
| PD | 23 (9.7) | 30 (11.9) | |
| 12-month survival rate (95% CI) (%) | 59.6 (52.3, 66.1) | 51.4 (44.3, 58.1) | |
| ORR (%) | 41.8 | 45.9 | 0.412 |
| DCR (%) | 90.3 | 88.1 | 0.443 |
- Abbreviations: RECIST, Response Evaluation Criteria In Solid Tumors; CR, complete remission; DCR, disease control rate; HR, hazards ratio; ORR, overall response rate; PD, progressive disease; PR, partial remission; SD, stable disease.
3.4 Safety results
In the current study, 534 patients were included in the safety set. AEs of any grade were reported in 97.7% of patients in the LP group and 99.6% of patients in the GP group (Table 4). SAEs occurred in 31.3% of patients in the LP group and 31.6% of patients in the GP group. A significantly lower proportion of patients in the LP group experienced AEs leading to treatment interruptions (10.9% vs. 26.4%, P < 0.001) or treatment termination (14.3% vs. 23.1%, P = 0.011). Though numerically more patients in the LP group experienced AEs leading to dose reductions, no statistical difference was found between the two groups (29.8% vs. 22.7%, P = 0.063).
| AEs | LP, n = 265, n (%) | GP, n = 269, n (%) | P value |
|---|---|---|---|
| Overall | 259 (97.7) | 268 (99.6) | 0.067 |
| Grade 3 and above | 181 (68.3) | 179 (66.5) | 0.712 |
| Incidence ≥ 5% | |||
| Neutropenia | 94 (35.5) | 76 (28.3) | 0.078 |
| Leucopenia | 62 (23.4) | 51 (19.0) | 0.244 |
| Bone marrow suppression | 41 (15.5) | 36 (13.4) | 0.539 |
| Anemia | 38 (14.3) | 84 (31.2) | < 0.001 |
| Hypopotassemia | 17 (6.4) | 14 (5.2) | 0.583 |
| Pulmonary infection | 14 (5.3) | 13 (4.8) | 0.846 |
| Thrombocytopenia | 4 (1.5) | 38 (14.1) | < 0.001 |
| Severe AEs | 83 (31.3) | 85 (31.6) | > 0.999 |
|---|---|---|---|
| AEs leading to treatment interruptions | 29 (10.9) | 71 (26.4) | < 0.001 |
| AEs leading to dose reductions | 79 (29.8) | 61 (22.7) | 0.063 |
|---|---|---|---|
| AEs leading to treatment termination | 38 (14.3) | 62 (23.1) | 0.011 |
The most frequently observed AE of any grade in the LP and GP group was anemia (71.3% vs. 80.7%, P = 0.015), followed by leucopenia (46.8% vs. 60.6%, P = 0.002) and neutropenia (43.0% vs. 55.0%, P = 0.006) (Supplementary Table S3). The most frequent grade ≥ 3 AE in the LP group was neutropenia (35.5%) followed by leucopenia (23.4%) and bone marrow suppression (15.5%) (Table 4). The most frequent grade ≥ 3 AE in the GP group was anemia (31.2%) followed by neutropenia (28.3%) and leucopenia (19.0%). The incidence of grade ≥ 3 anemia and thrombocytopenia in the LP group were significantly lower than in the GP group (Table 4).
3.5 The association between plasma cytokines and clinical outcomes of LSCC patients and observed dynamic changes in plasma cytokines in the LP group
To explore the association between the plasma cytokines levels and clinical outcomes of LSCC patients receiving LP treatment, we examined a panel of 45 cytokines in 86 patients randomly chosen from the LP group. Patient demographics, baseline features, and efficacy outcomes are shown in Supplementary Table S4. Figure 3A shows the expression levels of 45 cytokines in patients before initiation of LP therapy. Twenty-seven cytokines including TNF-α, interferon-gamma (IFN-γ), and IL-12p70 were differentially expressed in 45 patients who achieved PR and 41 patients who had SD (n = 27) or PD (n = 14) during the first efficacy evaluation (P < 0.05) (Figure 3B and Supplementary Figure S1). Univariate Cox regression analysis further showed that 15 cytokines including TNF-α, IFN-γ, IL-6, and IL-8 were significantly correlated with PFS (P < 0.05) (Figure 3C), with lower cytokine levels indicating better PFS (P < 0.05) (Supplementary Figure S2). Cytokines can function as effective but complex immune mediators. An essential feature of cytokine signaling is the degree of redundancy, in which multiple cytokines may display overlapping activities [17]. We, therefore, hypothesized that a group of cytokines, known as peripheral immune signature, might function synergistically. To better describe the function of these cytokines, the 45 cytokines were classified into different signatures according to their biological characterization, and corresponding signature scores were calculated. The scores of tumor necrosis factor signature and interferon signature in the LSCC patients before initiation of LP therapy were found to be negatively correlated with PFS; with lower cytokine signature scores indicating better PFS (Figure 4C).
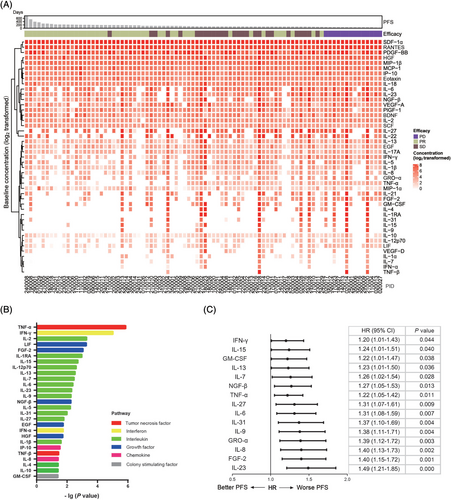
The association between baseline plasma cytokines and clinical outcomes of LSCC patients from the cisplatin plus Lipusu group. (A) Heatmap of 45 cytokines in 86 treatment-naïve LSCC patients from the cisplatin plus Lipusu group. Each row represents one cytokine and each column one patient. Patients were arranged based on the duration of PFS and were grouped by measurement of efficacy during the first evaluation. Effect_1st, efficacy during the first evaluation. Dark green, SD; yellow-green: PR; purple, PD. The cytokine levels are log2 transformed. Darker red indicates higher cytokine levels. (B) Twenty-seven cytokines were differentially expressed between 45 patients who achieved PR and 41 patients who had SD (n = 27) or PD (n = 14) during the first efficacy evaluation. Wilcoxon rank-sum test, P < 0.05. (C) Forest plot of correlation between 15 cytokine levels and PFS of 86 patients from the cisplatin plus Lipusu group. Abbreviations: PFS, progression-free survival; PR, partial response; SD, stable disease; PD, progression disease
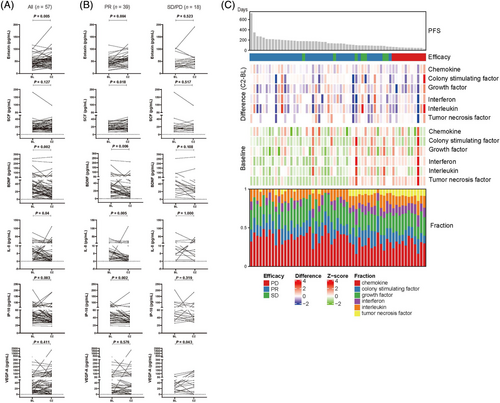
Dynamic changes of plasma cytokines and cytokine signatures at baseline and after two cycles of cisplatin plus Lipusu therapy. (A) Plasma cytokine levels at baseline and after two cycles of cisplatin plus Lipusu therapy. (B) Plasma cytokine levels at baseline and after two cycles of cisplatin plus Lipusu therapy in 39 patients who achieved PR and 18 patients who had SD or PD. (C) Complex heatmap of plasma cytokine signatures at baseline and the dynamic changes after two cycles of cisplatin plus Lipusu therapy in 57 patients. Each row represents one cytokine signature and each column one patient. Patients were arranged based on the duration of PFS and were grouped by measurement of efficacy during the first evaluation. Effect_1st means efficacy during the first evaluation. Baseline shows the heatmap of cytokine signatures scores at baseline. All data were standardized by the Z-score method according to row. Difference (C2-BL) was calculated as the difference score of each cytokine signature between two cycles of cisplatin plus Lipusu therapy and baseline. Fraction was calculated as the percentage of each cytokine signature in all six signatures in one patient. Abbreviations: PFS, progression-free survival; BL, baseline; C2, two cycles of cisplatin plus Lipusu therapy; PR, partial response; SD, stable disease; PD, progression disease
To explore the potential of a subsequent combination of immunotherapy in the LP group, we examined the plasma cytokine levels in 57 LSCC patients with the paired samples of baseline and first efficacy evaluation. The plasma levels of eotaxin (CCL11) were significantly elevated (P = 0.005) while BDNF (P = 0.002), IL-8 (CXCL8) (P = 0.004) and IP-10 (CXCL10) (P = 0.003) were noticeably reduced after the two cycles of LP therapy versus baseline (Figure 4A). Moreover, subgroup analysis further showed that the levels of plasma CCL11 (P = 0.004) and SCF (P = 0.018) were significantly elevated while brain-derived neurotrophic factor (BDNF) (P = 0.006), IL-8 (P = 0.005) and CXCL10 (P = 0.002) were noticeably declined after the two cycles of LP therapy versus baseline in 39 patients who achieved PR (Figure 4B). Meanwhile, the levels of plasma VEGF-A were significantly elevated after two cycles of LP therapy versus baseline in 18 patients who had SD or PD (P = 0.043) (Figure 4B). The various dynamic changes in plasma cytokine levels by PFS achieved are shown in Figure 4C.
4 DISCUSSION
This randomized clinical trial demonstrated that cisplatin/lipusu as first-line treatment for locally advanced or metastatic LSCC achieved comparable ORR (41.8% vs. 45.9%), median PFS (5.2 vs. 5.5 months), and median OS (14.6 vs. 12.5 months) compared to combination of cisplatin/gemcitabine which is the currently recommended first-line treatment for LSCC [25, 26], thus, suggesting cisplatin/lipusu as an alternative effective treatment regimen for locally advanced or metastatic LSCC patients. These results were similar with another phase II study that compared nab-paclitaxel/carboplatin and carboplatin/gemcitabine, with an ORR of 42% vs. 27% (P > 0.05), median PFS of 6.7 vs. 5.8 months (P = 0.143), and median OS of 11.6 vs. 14.4 months (P = 0.846) [27]. These findings may further confirm the fact that there were no significant differences among the third-generation cytotoxic agents regarding therapeutic efficacy [28], therefore, the key to select an optimal chemotherapeutic regimen could be based on the potential safety profiles.
Notably, our study demonstrated that the LP regimen was well-tolerated, with fewer treatment interruptions (10.9% vs. 26.4%) or terminations (14.3% vs. 23.1%) compared to the GP regimen. Moreover, the incidences of AEs (except hair loss) in the LP group were significantly lower than those of the GP group. In addition, the incidence of grade ≥3 anemia (14.3% vs. 31.2%) and thrombocytopenia (1.5% vs. 14.1%) in the LP group was significantly lower than that of the GP group. Thus, our results suggest a new option for patients with locally advanced or metastatic LSCC, which could be significantly safer and less toxic.
As accumulating evidences indicate that circulating cytokines may serve as predictive biomarkers for the efficacy of systemic therapy [18-20], we also investigated the association between the baseline levels of cytokines and clinical outcomes of LP treatment to identify potential responders to LP treatment. The current study demonstrated that baseline levels of 14 diverse cytokines such as TNF-a, IFN-γ, and IL-12p70 were differentially expressed in patients who achieved PR as compared to those who did not. In addition, a spectrum of cytokines showed a significantly negative correlation with PFS. These cytokines included Th1 cytokines (TNF-a, IFN-γ, and granulocyte-macrophage colony-stimulating factor [GM-CSF]), Th2 cytokines (IL-13 and IL-6), and others (IL-9, IL-23, IL-27) (Supplementary Table S5). Our findings supported the hypothesis that plasma cytokines can act as biomarkers for predicting the clinical benefits of patients who have received chemotherapy. Therefore, it would be of clinical significance if further studies could be conducted to establish a predictive cytokine signature to facilitate the proper selection of locally advanced or metastatic LSCC patients for LP therapy and other systemic therapy. However, we currently only performed cytokine analysis in the LP group, not yet in the GP group. Additionally, a similar assessment for the GP group is needed for further validation.
Notably, the current study also found that the levels of a spectrum of cytokines, including BDNF, IL-8 and CXCL10 were noticeably decreased after two cycles of LP therapy. Interestingly, patients with lower baseline plasma IL-8 levels or whose IL-8 levels significantly declined after LP therapy displayed significantly better clinical outcomes. IL-8 is a versatile cytokine that can participate in the pathogenesis of various inflammatory diseases and cancers [29]. IL-8 exerts pleiotropic functions, such as regulation of the recruitment of neutrophils, promotion of angiogenesis, and stimulation of tumor cell proliferation, invasion as well as metastasis [29]. Recent studies have demonstrated that elevated serum IL-8 was associated with reduced clinical benefit of ICIs [30-32]. Therefore, our study may also partially explain the success of the combination of chemotherapy and ICIs. It should be noted that most of these trials have selected paclitaxel-based chemotherapy to be used for the combination therapies [33-35]. Therefore, paclitaxel-based chemotherapy might create a favorable tumor microenvironment, not only by releasing tumor antigens and inducing the activation of immune cells, including dendritic cells (DCs), natural killer cell (NK) and tumor-specific cytotoxic T cells [36, 37], but also by modulating the expression levels of cytokines [38].
One of the limitations of the current study is that all patients were recruited from China. Therefore, these findings should be further validated before generalization across broader population profiles. Furthermore, there was a lack of evaluation of the levels of cytokines evaluation in patients who received the GP regimen. The association between the levels of cytokines and clinical outcomes as well as the potential impact of GP on the dynamic changes of cytokines remain unclear. Thirdly, other circulating biomarkers, such as circulating tumor cells and circulating-free tumor DNA were not included in the analyses. Finally, in our study, high proportions of patients with stage IIIB diseases were enrolled (33.96% in the LP group while 32.34% in GP group) and it might have an impact on survival outcomes. Actually, the median OS of both groups extended 1 year, which was longer than historical data [25]. However, the distributions of stage IIIB diseases were well-balanced between the two groups, therefore, we expect that this would not significantly influence the survival outcomes analyses.
In conclusion, this study showed that the LP regimen had similar PFS, OS, ORR and DCR as the GP regimen for patients with locally advanced or metastatic LSCC, but also demonstrated a more favorable in toxicity profiles, thereby supporting the application of the LP regimen as an effective and safe alternative first-line treatment. The study also clearly demonstrated the potential impact of LP treatment on the levels of plasma cytokines and identified a spectrum of cytokines that were found to be associated with clinical benefit in patients who received LP.
DECLARATIONS
ACKNOWLEDGMENTS
This study was sponsored by Nanjing Luye Pharmaceutical Co. Ltd, Nanjing, China. Cytokines detection was conducted by Wuxi Genecast Biotechnology Co. Ltd, Wuxi, China. We thank the patients who participated in this study, as well as their families and caregivers.
AUTHORS’ CONTRIBUTIONS
CCZ was the leading principal investigator of this study, contributed to study conception, study design, data analysis and interpretation, manuscript drafting, and editing. JZ, YYP, QS, GJZ, LYJ, XRD, KSG, HJW, XCZ, NY, YPL, JPX, TNY, MP, YS, YF, JWC, GYC, WT, AMZ, QSG, GQZ, ZPW, JXH, WXY, XHW, KC, XHH, CHH, LY, DJ, GFW, JFL, GHY, JLL, were involved in patient recruitment and data acquisition. WMX, WHZ did the literature search, research data analysis and interpretation. LHW analyzed the correlation between plasma cytokines and treatment outcomes. JLB did the data statistical analysis. All authors were involved in the drafting, reviewed the manuscript. All authors read and approved the final manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This trial was conducted in accordance with the Declaration of Helsinki. The independent ethics committee or institutional review board of each participating study center approved the protocol and all amendments. All participants provided written informed consent before enrollment. The trial number was NCT02996214.
CONSENT FOR PUBLICATION
Not applicable.
COMPETING INTERESTS
This study was sponsored by Nanjing Luye Pharmaceutical Co. Ltd, Nanjing, Jiangsu, China. WMX and WHZ are employees of Nanjing Luye Pharmaceutical Co. Ltd, Nanjing, Jiangsu, China. LHW is an employee of Wuxi Genecast Biotechnology Co. Ltd, Wuxi, Jiangsu, China. The other authors have no conflicts of interest to declare.
FUNDING
This study was sponsored by Nanjing Luye Pharmaceutical Co. Ltd, Nanjing, China, and supported in part by grants from Shanghai Key disciplines of Respiratory (No. 2017ZZ02012), and Shanghai Major Diseases Multidisciplinary Cooperation Diagnosis and Treatment Construction Project.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the reported findings of this study is available on request from the corresponding author. The data was not made publicly available due to privacy or ethical restrictions.




