Clinical outcomes of second-line treatment following first-line VEGFR-TKI failure in patients with metastatic renal cell carcinoma: a comparison of axitinib alone and axitinib plus anti-PD-1 antibody
Abbreviations
-
- VEGFR-TKI
-
- vascular endothelial growth factor receptor-tyrosine kinase inhibitor
-
- ICI
-
- immune checkpoint inhibitor
-
- PD-1
-
- programmed cell death protein 1
-
- mRCC
-
- metastatic renal cell carcinoma
-
- PFS
-
- progression-free survival
-
- OS
-
- overall survival
-
- ORR
-
- objective response rate
-
- IMDC
-
- International Metastatic Renal Cell Carcinoma Database Consortium
-
- AEs
-
- adverse events
Dear editor,
The prognosis of metastatic renal cell carcinoma (mRCC) has been significantly improved with the development and widespread use of vascular endothelial growth factor (VEGF) pathway inhibitors and mammalian target of rapamycin (mTOR) pathway inhibitors [1]. For the past decade, the standard of care utilized in the first-line setting was VEGF-targeted therapies. Recently, the mRCC treatment landscape has rapidly changed with the exploration of combinations of immune checkpoint inhibitors (ICIs) and anti-VEGF agents [2, 3]. The Keynote-426 trial demonstrated both progression-free survival (PFS) and overall survival (OS) advantages of axitinib plus pembrolizumab over sunitinib for untreated advanced RCC patients [2]. Based on these results, axitinib plus pembrolizumab, cabozantinib plus nivolumab, lenvatinib plus pembrolizumab, and nivolumab plus ipilimumab have been recommended as first-line treatment in recent guidelines [3]. However, axitinib plus pembrolizumab as first-line treatment for mRCC is only approved in limited countries, in which China is not included. Vascular endothelial growth factor receptor-tyrosine kinase inhibitors (VEGFR-TKIs) remain a recommended option as first-line therapy in China and in other countries. Nivolumab, cabozantinib, or axitinib remains the standard care for patients who failed first-line therapy with VEGFR-TKI. Considering that axitinib plus anti-programmed cell death protein 1 (PD-1) antibody was expected to have a better oncological outcome than axitinib alone owing to first-line study results [2], the subsequent therapy algorithm after first-line VEGFR-TKI failure needs to be redefined. So far, there is no research published on VEGFR-TKI and ICI combination therapy after first-line VEGFR-TKI failure.In this context, the present retrospective multi-center study was aimed to compare the objective response rate (ORR), PFS, OS, and toxicities between axitinib plus anti-PD-1 antibody and axitinib alone for mRCC after first-line VEGFR-TKI failure.
Clinical data were retrieved from the electronic medical records of mRCC patients treated at 5 participating centers between October 2015 and October 2020. The patient selection and assessments are detailed in the Supplementary file of Patients and Methods. A total of 255 patients were included in this study, of whom 116 received axitinib plus anti-PD-1 antibody combination therapy, and 139 received axitinib alone (Supplementary Figure S1). Anti-PD-1 antibody agents used in this study were used off label, including pembrolizumab (n = 32, 27.6%), nivolumab (n = 6, 5.2%), toripalimab (n = 42, 36.2%), sintilimab (n = 32, 27.6%), and tislelizumab (n = 4, 3.4%). All patients were aware of this situation and signed informed consent before treatment. Supplementary Table S1 shows the baseline characteristics of these 255 patients.
The best responses of patients are shown in Supplementary Table S2. According to Response Evaluation Criteria in Solid Tumors version 1.1, only 2 patients in the combination group had complete response (CR), while none in the axitinib group had CR. Meanwhile, 37 patients in the combination group and 28 in the axitinib group were determined to have partial response (PR). The ORR in the combination group was significantly higher than that in the axitinib group (33.6% vs. 20.1%, P = 0.015).
After a median follow-up period of 25.7 (95% confidence interval [CI] = 15.4-36.0) months from the start of second-line treatment, the median PFS was 11.7 (95% CI = 9.2-14.2) months for patients treated with combination therapy and 7.5 (95% CI = 5.2-9.8) months for patients treated with axitinib alone. The PFS of the combination group was significantly longer than that of the axitinib group (P = 0.002) (Figure 1A). The median OS was not reached in the combination group and was 21.4 (95% CI = 13.7-29.1) months for patients treated with axitinib alone (Figure 1B). No significant difference in OS was found (P = 0.201).
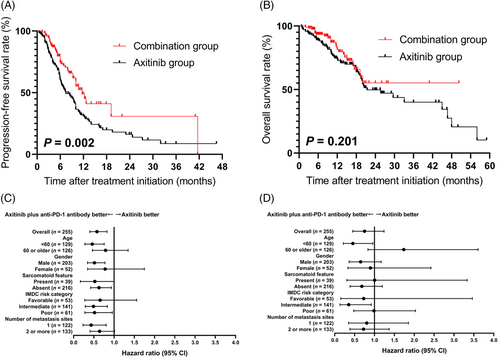
PFS, OS, and subgroup analysis of 255 mRCC patients who received axitinib alone or axitinib plus anti-PD-1 antibody following first-line VEGFR-TKI failure. A. Kaplan-Meier PFS curves of patients treated with combination therapy and axitinib alone. B. Kaplan-Meier OS curves of patients treated with combination therapy and axitinib alone. C. Subgroup analysis of PFS. D. Subgroup analysis of OS
Abbreviations: PFS, progression-free survival; OS, overall survival; mRCC, metastatic renal cell carcinoma; PD-1, programmed cell death protein 1; VEGFR-TKI, vascular endothelial growth factor receptor-tyrosine kinase inhibitor; IMDC, International Metastatic Renal Cell Carcinoma Database Consortium.
Figure 1C shows the subgroup analysis of PFS with respect to baseline characteristics and International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) classification using Cox proportional hazards model. Factors favoring combination therapy included IMDC intermediate risk (hazard ratio [HR] = 0.490, 95% CI = 0.288-0.833) and poor risk (HR = 0.516, 95% CI = 0.279-0.956). However, in the subgroup analysis of OS (Figure 1D), the advantage of combination therapy was only observed in IMDC intermediate-risk mRCC (HR = 0.346, 95% CI = 0.133-0.905). Furthermore, the combination therapy significantly prolonged PFS in patients with (HR = 0.407, 95% CI = 0.167-0.995) or without sarcomatoid features (HR = 0.626, 95% CI = 0.421-0.931).
Next, we evaluated the associations between clinical characteristics and survival. IMDC risk, discontinuation of first-line therapy due to AEs, and second-line regimen were significantly associated with PFS (Supplementary Table S3); IMDC risk was identified as an independent predictor of OS (Supplementary Table S4).
At the time of data analysis, in the combination therapy group, adverse events (AEs) led to discontinuation of either drug in 11 (9.5%) patients, discontinuation of both drugs in 6 (5.2%) patients, and dose reduction of axitinib in 9 (7.8%) patients. While in the axitinib group, 17 (12.2%) patients required dose reduction, and 10 (7.2%) needed drug discontinuation. Major treatment-related adverse events are presented in Supplementary Table S5.
In the present study, axitinib plus anti-PD-1 antibody showed ORR and PFS benefits over axitinib alone in mRCC patients. One concern is whether the benefits of the combination therapy could be observed across all IMDC risk categories. We found that the combination therapy prolonged PFS in the IMDC intermediate- or poor-risk subgroup, but not in the favorable-risk subgroup. However, the OS benefit was only observed in the IMDC intermediate-risk subgroup, probably due to the small number of patients at IMDC poor risk and short follow-up period. According to the subgroup analysis of the Keynote-426 study [4], pembrolizumab plus axitinib prolonged both OS (HR = 0.52, 95% CI = 0.37-0.74) and PFS (HR = 0.67, 95% CI = 0.53-0.85) in patients with IMDC intermediate or poor risk, which were similar to our findings. Based on these results, we speculate that patients with IMDC favorable risk mRCC might have no added benefit from the combination therapy after first-line TKI failure given the high cost of ICI-based therapy and immune-related AEs [5, 6], while patients with IMDC intermediate- or poor-risk mRCC might benefit most from the combination therapy.
In patients with mRCC treated with TKIs, sarcomatoid features had been reported to be associated with poor prognosis [7], but recent studies suggested that ICI-based combination therapy could dramatically improve the outcomes of these patients [4, 8]. The subgroup analysis of the Keynote-426 study [4] showed a benefit in PFS (HR = 0.54, 95% CI = 0.29-1.00) with combination therapy compared to sunitinib alone in patients with sarcomatoid features, which was similar to our results.
Some side effects of combination therapy seemed to add up numerically [2, 9, 10]. The rate of grade ≥3 liver enzyme elevation was significantly higher in the combination group than in the axitinib group, which was consistent with historic comparisons such as the Keynote-426 study [2].
The limitation of this study was represented by the relatively short follow-up, the retrospective design, and selection bias. The OS data were not mature in the combination group. In addition, patients who received cabozantinib, ICI alone, or ICI combination as second-line therapy were not included in this study because cabozantinib and anti-CTLA-4 antibodies were not commercially available in China during the study period. Nevertheless, the ideal order of sequential therapy remains unclear, and the combinational use of axitinib plus anti-PD-1 antibody in this setting should be validated in future prospective studies as a standard of care.
In conclusion, among mRCC patients with first-line VEGFR-TKI failure, second-line treatment with axitinib plus anti-PD-1 antibody may prolong PFS and increase ORR as compared with axitinib alone.
ACKNOWLEDGEMENTS
Not applicable.
AUTHORS’ CONTRIBUTIONS
J.H., S.W., J.G., Q.W. and W.X. designed the research. W.X., S.W., J.G., Q.W. and W.C. provided executive support and data surveillance and performed the patient selection process. Y.W., H.Z., X.H., P.W., W.C. and Y.Y. acquired and analyzed the data. J.H., Y.W., H.Z., X.H., P.W., W.C. and Y.Y. wrote the manuscript. H.Z., J.Z., W.K., J.Z. and Y.H. revised the manuscript. All authors reviewed the manuscript critically and approved the content.
COMPETING INTERESTS
The authors declare that no competing interests exist.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was conducted in accordance with the ethical standards of the Declaration of Helsinki and approved by the independent ethics committee of all 5 participating centers.
FUNDING
This study was supported by Shanghai Science and Technology Commission Research Project (21ZR1438900) and the Incubating Program for Clinical Research and Innovation of Renji Hospital (PYXJS16-008, PYIII20-07).
CONFLICT OF INTEREST DISCLOSURES
The authors have declared no conflicts of interest.




