Evaluation of renal cancer progression in a mouse model of heart failure
Abbreviations
-
- CO
-
- cardiac output
-
- FS
-
- fractional shortening
-
- HF
-
- heart failure
-
- LVEF
-
- left ventricular ejection fraction
-
- MI
-
- myocardial infarction
-
- Renca-luc
-
- luciferase-expressing Renca cell line
-
- TAC
-
- transverse aortic constriction
-
- TL
-
- tibia length
Heart failure (HF) is a globally prevalent health problem, with high mortality and morbidity despite advanced pharmacological and device-based treatment options. Accumulating epidemiological data suggest that patients with preexisting HF are more susceptible to develop incident cancer when compared to subjects free of HF, which might be explained by shared risk factors including age, hypertension, obesity, diabetes, and smoking [1-3].
Recent preclinical work has shown that HF presented as a pro-oncogenic condition. Meijers et al. [4] demonstrated for the first time that HF induced by myocardial infarction (MI) resulted in a robust increase of intestinal tumor load in mice spontaneously developing multiple intestinal neoplasia induced by adenomatous polyposis coli mutations, associated with upregulation of secreted circulatory proteins such as SerpinA3. Koelwyn et al. [5] reported that MI-induced HF accelerated breast cancer growth in mice by enhancing circulating Ly6Chi monocytes and their recruitment to tumors, which restricted T cell infiltration and antitumoral immune responses. Furthermore, Avraham et al. [6] provided evidence that cardiac remodeling induced by transverse aortic constriction (TAC) in mice also enhanced breast and lung cancer growth, again explained by increased expression of secreted factors from the failing heart.
These experimental data are in line with clinical observations that HF is associated with increased incidences of various cancer types [1]. Two studies using Danish National Patient Registry (https://sundhedsdatastyrelsen.dk/da/registre-og-services/om-de-nationale-sundhedsregistre/sygedomme-laegemidler-og-behandlinger/landspatientregisteret) reported that HF was associated with increased risks of overall and multiple type-specific cancers, including lung, breast, colon, and liver cancers, as well as renal cancer, which was significantly increased by 65% after adjustment for age and sex [7, 8]. Despite this overall clear association, it remains unknown whether it is a generic phenomenon or specific to particular cancer types. To evaluate the direct effects of HF on renal cancer progression, we herein studied a mouse model of renal cancer to determine whether HF would accelerate renal cancer growth.
The full experimental protocol is illustrated in Figure 1A, and the details of methods can be found in the Supplementary materials and methods. In brief, 8-week-old male BALB/c mice were subjected to MI surgery to provoke HF. One and 5 weeks after surgery, echocardiography was performed to assess the cardiac structure and function. A well-established orthotopic renal cancer model was utilized. To monitor tumor growth in vivo, we first generated a stable luciferase-expressing renal cancer cell line (Renca-luc). Serial dilutions of cell suspension resulted in a strong correlation between cell number and the measured bioluminescent signals (R2 = 0.928, P < 0.001; Figure 1B and 1C). Two weeks after MI/sham surgery, 2 × 103 Renca-luc cells were orthotopically implanted into the right kidney. The tumor growth was monitored using an IVIS 100 Imaging System, and the bioluminescent signals emitted by tumor cells were continuously increasing over time (Figure 1D).
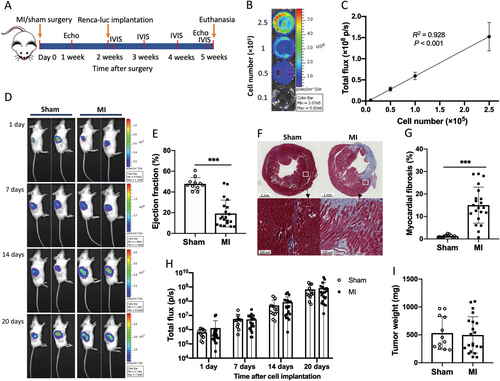
A total of 48 mice were allocated to MI or sham surgery. Six mice died during surgical and echocardiography procedures. No death was observed during the implantation of cancer cells. One sham mouse with abnormal cardiac function was excluded from the analysis. Eight mice failed to develop tumors (4 mice in each group). The final group sizes were 21 in the MI group and 12 in the sham group.
Echocardiography measurements at 1 week after MI surgery revealed a clear reduction in left ventricular ejection fraction (LVEF), fractional shortening (FS), and cardiac output (CO): LVEF, 19.3 ± 12.9% vs. 47.9 ± 6.1%; FS, 8.9 ± 6.5% vs. 23.7 ± 3.7%; CO, 5.4 ± 2.8 vs. 10.5 ± 1.8 mL/min in MI versus sham (all P < 0.001; Figure 1E, Supplementary Figure S1A and S1B). Longitudinal analyses of cardiac function demonstrated no changes in the sham group, while 5 weeks after MI, mice showed slightly increased CO, progressive LV dilatation with increased diameters, but with sustained decreases in LVEF and FS compared to the 1-week data (Supplementary Figure S1C-E). Myocardial fibrosis was determined by Masson trichrome staining and was significantly increased in the MI group compared to the sham group (15.0 ± 8.0% vs. 1.3 ± 0.6%, P < 0.001; Figure 1F and 1G). Accordingly, cardiac hypertrophy was observed in HF mice, as reflected by enlarged atria and ventricles with 2.1-fold and 20% mass increases, respectively (both P < 0.001; Supplementary Figure S1F and S1G). Congestion was not observed, determined by no difference in lung, liver and left kidney weights between the sham and MI groups (Supplementary Figure S1H-J).
There was no significant difference in bioluminescent signals between sham and HF mice at each time point (Figures 1H). Consistently, MI mice had comparable renal tumor weights as well as kidney volumes compared to sham mice (Figure 1I and Supplementary Figure S1K), which indicated HF had neutral effects on renal cancer growth.
Patients with renal cell carcinoma may develop metastases, with lung and liver being the predominant sites [9, 10]. We therefore checked the presence of metastases in our mice, but no metastatic nodules were observed on the surface of the lung or liver during the euthanasia. This was further confirmed by hematoxylin and eosin staining (Supplementary Figure S1L). There was no significant luciferase expression detected in the liver and lung by qPCR (all Cq > 34, data not shown).
Multiple studies have highlighted the potential importance of cardiovascular disease and HF for cancer development, providing evidence for accelerated growth of colon, breast, and lung cancers in mice, which was shown to be mediated by circulating proteins or immune system alterations and was consistent with increasing risks of cancer incidence in HF patients [4-6]. In the current study, HF did not accelerate renal cancer progression, which was demonstrated by the comparable emitted bioluminescent signal, tumor weight, kidney volume, and the absence of distant metastases.
Our study, together with previous evidence, suggests that the effects of HF on tumor growth are not generic, and likely the underlying mechanisms might be specific for HF subforms, cancer types, and/or experimental models. In our previous study, we evaluated factors of the cardiac secretome, in which some had mitogenic effects on colon tumor cell lines [4]. In line, the identified elevated HF-specific secreted proteins, including ceruloplasmin, Serpin A1 and A3, had no effects on the proliferation of Renca cells in vitro (data not shown) [4]. Additionally, it is noteworthy that although both MI and TAC accelerated breast cancer growth, the altered immune reprogramming triggered by MI with further anti-immune response of tumor cells was not observed in TAC [5, 6]. This underscores that the pathophysiological mechanisms will likely vary according to HF etiology.
It is also important to consider that mouse models are substitutes for human cancer. Interestingly, Schwartz et al. [8] reported that the incident rate of various cancer types, including renal cancer, raised in HF patients compared to control subjects from the general population, but these risks were mainly driven by the presence of comorbidities such as ischemic heart disease, diabetes, chronic obstructive pulmonary disease, and chronic kidney disease. Typically, in rodent animal studies, the animals are young and free of co-morbid conditions. This fact may partly explain that the association between HF and renal cancer observed in clinical studies cannot be translated in an animal study, on top of species differences. In consequence, effects of comorbidities on triggering cross-disease communication should be taken into consideration in future studies.
In summary, we demonstrate that MI-induced HF is not associated with accelerated tumor growth in an orthotopic renal cancer model. The effects of HF on cancer growth might be specific for cancer types and/or experimental models, and we caution against generalizations with regards to the described interaction between HF and tumor growth.
DECLARATIONS
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The experimental protocol was approved by the Animal Ethical Committee of University of Groningen (IvD number: 198647-01-001) and was conducted according to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health.
CONFLICT OF INTEREST
The University Medical Center Groningen (UMCG), which employs the authors, has received research grants and/or fees from AstraZeneca, Abbott, Bristol-Myers Squibb, Novartis, Novo Nordisk, and Roche. Dr. de Boer received speaker fees from Abbott, AstraZeneca, Bayer, Novartis, and Roche.
ACKNOWLEDGMENTS
Canxia Shi is supported by a scholarship from the China Scholarship Council [CSC number: 201806170057]. This work was supported by a grant from the European Research Council (ERC CoG 818715, SECRETE-HF). Further support was obtained from grants from the Netherlands Heart Foundation (CVON SHE-PREDICTS-HF, grant 2017-21; CVON RED-CVD, grant 2017-11; CVON PREDICT2, grant 2018-30; and CVON DOUBLE DOSE, grant 2020B005), and by a grant from the leDucq Foundation (Cure PhosphoLambaN induced Cardiomyopathy (Cure-PLaN). Dr. Meijers is supported by the Mandema-Stipendium of the Junior Scientific Masterclass 2020-10, University Medical Center Groningen. We would like to thank Tim R. Eijgenraam for his experimental assistance.
AUTHORS' CONTRIBUTIONS
RdB and HS designed and supervised this project. CS and ES conducted most animal experiments with the help from JA, SdW and VB. CS and WM performed the data analysis. CS wrote the manuscript which was revised and proved by all authors.




