Advancing to the era of cancer immunotherapy
Abstract
Cancer greatly affects the quality of life of humans worldwide and the number of patients suffering from it is continuously increasing. Over the last century, numerous treatments have been developed to improve the survival of cancer patients but substantial progress still needs to be made before cancer can be truly cured. In recent years, antitumor immunity has become the most debated topic in cancer research and the booming development of immunotherapy has led to a new epoch in cancer therapy. In this review, we describe the relationships between tumors and the immune system, and the rise of immunotherapy. Then, we summarize the characteristics of tumor-associated immunity and immunotherapeutic strategies with various molecular mechanisms by showing the typical immune molecules whose antibodies are broadly used in the clinic and those that are still under investigation. We also discuss important elements from individual cells to the whole human body, including cellular mutations and modulation, metabolic reprogramming, the microbiome, and the immune contexture. In addition, we also present new observations and technical advancements of both diagnostic and therapeutic methods aimed at cancer immunotherapy. Lastly, we discuss the controversies and challenges that negatively impact patient outcomes.
Abbreviations
-
- ACTs
-
- adoptive cell therapies
-
- AMP
-
- adenosine monophosphate
-
- APCs
-
- antigen-presenting cells
-
- Arg1
-
- arginase-1
-
- ASCO
-
- American Society of Clinical Oncology
-
- ATP
-
- adenosine triphosphate
-
- BAs
-
- bile acids
-
- bTMB
-
- tumor mutational burden in blood
-
- CAR-Ms
-
- chimeric antigen receptor macrophages
-
- CAR-T
-
- chimeric antigen receptor T cells
-
- Cebpb
-
- CCAAT enhancer binding protein beta
-
- cfDNA
-
- circulating cell-free DNA
-
- cGAS
-
- cyclic guanosine monophosphate-adenosine monophosphate synthase
-
- CRC
-
- colorectal cancer
-
- CTCs
-
- circulating tumor cells
-
- ctDNA
-
- circulating tumor DNA
-
- CTLA-4
-
- cytotoxic T lymphocyte-associated antigen 4
-
- CTLs
-
- cytotoxic T lymphocytes
-
- CYT
-
- cytolytic activity
-
- DAMPs
-
- damage-associated molecular patterns
-
- DCs
-
- dendritic cells
-
- dMMR
-
- mismatch repair-deficient
-
- eATP
-
- adenosine triphosphate in the extracellular space
-
- EOMES
-
- eomesodermin
-
- ESMO
-
- European Society for Medical Oncology
-
- FAO
-
- fatty acid oxidation
-
- FDA
-
- the Food and Drug Administration
-
- FMT
-
- fecal microbiota transplantation
-
- GBM
-
- glioblastoma multiforme
-
- GEP
-
- gene expression profile
-
- GM-CSF
-
- granulocyte-macrophage colony stimulating factor
-
- HLA
-
- human leukocyte antigen
-
- HMAs
-
- hypomethylating agents
-
- HMGB1
-
- high mobility group box 1
-
- HNSCC
-
- head and neck squamous cell carcinoma
-
- HPD
-
- hyperprogressive disease
-
- HSV-1
-
- type 1 herpes simplex virus
-
- HTAN
-
- The Human Tumor Atlas Network
-
- ICB
-
- immune checkpoint blockade
-
- ICIs
-
- immune checkpoint inhibitors
-
- IDO
-
- indoleamine-pyrrole 2,3-dioxygenase
-
- IGF1R
-
- insulin like growth factor 1 receptor
-
- IFNs
-
- interferons
-
- IL
-
- interleukin
-
- irAEs
-
- immune-related adverse events
-
- ITIM
-
- immunoreceptor tyrosine-based inhibitory motif
-
- JAK
-
- janus kinase
-
- LAG-3
-
- lymphocyte activation gene-3
-
- LUSC
-
- lung squamous cell carcinoma
-
- mAb
-
- monoclonal antibody
-
- MDSCs
-
- myeloid-derived suppressor cells
-
- MerTK
-
- c-mer proto-oncogene tyrosine kinase
-
- mIHC
-
- multiplex immunohistochemistry
-
- MRR
-
- mismatch repair
-
- MSI
-
- microsatellite instability
-
- MSI-H
-
- high microsatellite instability
-
- MSI-L
-
- low microsatellite instability
-
- MSS
-
- microsatellite stability
-
- mTOR
-
- mechanistic target of rapamycin kinase
-
- NGS
-
- next-generation sequencing
-
- NICHE
-
- Nivolumab, Ipilimumab and COX2-inhibition in Early Stage Colon Cancer: an Unbiased Approach for Signals of Sensitivity
-
- NK cells
-
- natural killer cells,
-
- NKT cells
-
- natural killer T cells
-
- NSCLC
-
- non-small cell lung cancer
-
- nsSNVs
-
- Nonsynonymous single-nucleotide variants
-
- OS
-
- overall survival
-
- OVs
-
- oncolytic viruses
-
- OXPHOS
-
- oxidative phosphorylation
-
- p21
-
- cyclin-dependent kinase inhibitor 1
-
- PAMPs
-
- pathogen-associated molecular patterns
-
- PD-1
-
- programmed death 1
-
- PFS
-
- progression-free survival
-
- PKA
-
- protein kinase ANF-kB: nuclear factor-κB
-
- PI3K
-
- phosphoinositide 3-kinase
-
- pMMR
-
- mismatch repair - proficient
-
- PTMs
-
- posttranslational modifications
-
- SH2
-
- src homology 2
-
- SHP-1
-
- src homology 2 domain-containing protein tyrosine phosphatase 1
-
- SHP-2
-
- src homology 2 domain-containing protein tyrosine phosphatase 2
-
- SIGLEC-10
-
- sialic acid binding Ig-like lectin 10
-
- SIGLEC-15
-
- sialic acid binding Ig-like lectin 15TCAA: T cell activity array
-
- SIRPα
-
- signal regulatory protein α
-
- ST2
-
- suppression of tumorigenicity 2
-
- STAT
-
- signal transducer and activator of transcription
-
- 3D
-
- three-dimensional
-
- TAAs
-
- tumor-associated antigens
-
- TAMs
-
- tumor-associated macrophages
-
- TCGA
-
- The Cancer Genome Atlas
-
- TCR
-
- T cell receptor
-
- TCR-T
-
- TCR-engineered T cells
-
- Th cells
-
- CD4+ helper T cells
-
- Tfh cells
-
- follicular helper T cells
-
- TIM-3
-
- T cell immunoglobulin and mucin domain-containing protein 3
-
- TIGIT
-
- T cell immunoglobulin and ITIM domain
-
- TMB
-
- tumor mutational burden
-
- TMB-H
-
- high tumor mutational burden
-
- TILs
-
- tumor-infiltrating lymphocytes
-
- TME
-
- tumor microenvironment
-
- Tregs
-
- regulatory T cells
-
- T-VEC
-
- talimogene laherparepvec
-
- VEGF
-
- vascular endothelial growth factorTLSs: tertiary lymphoid structures
-
- VISTA
-
- V-domain immunoglobulin suppressor of T cell activation
1 BACKGROUND
Cancer is one of the biggest medical problems limiting the life span of humans and is also one of the leading challenges to overcome for patients and doctors worldwide. During the last century, scientists have developed diversified treatments for cancer, including surgery, radiotherapy, and chemotherapy. Although great progresses have been achieved via these conventional strategies, many cancer patients are still facing the following two major issues: i) late diagnosis leading to advanced-stage disease due to nonspecific clinical symptoms or inadequate screening facilities, and ii) low efficacy of conventional treatments due to the rapid spread of cancer and/or drug resistance.
In recent years, immunotherapy has become a major focus among tumor treatments, as it provides insights for further prolonging the overall survival (OS) of patients while improving the patients’ quality of life [1, 2]. The history of immunotherapy can be traced back to 1893 when the American surgeon William Coley found that live or inactivated bacteria could cause remission in sarcomas [3]. However, it was not until the 1980s and 1990s that scientists discovered the interaction between immune cells and melanoma, and conceptualized the idea of cancer immunotherapy[4, 5]. Based on the notable benefits resulting from the clinical utilization of new treatments such as cancer vaccines and immune checkpoint inhibitors (ICIs) [6-8], Science chose ‘cancer immunotherapy’ as the ‘breakthrough of the year’ in 2013 [9]. Furthermore, the discovery of programmed death 1 (PD-1) and the targeting of cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) in cancer led to the Nobel Prize for Physiology or Medicine honoring the scientists Tasuku Honjo and James Allison in 2018 [10].
Tumors possess immunogenicity characteristics similar to those of other pathogenic agents while also reserving many specific biological reactions. The process of antitumor immunity requires the participation of various immune cells. In most cases, the first step in antitumor immunity is the exposure of tumor-associated antigens (TAAs) to antigen-presenting cells (APCs), particularly dendritic cells (DCs) and macrophages [11]. In complex with human leukocyte antigen (HLA) class I and II molecules, TAAs are presented by DCs to CD8+ T cells (cytotoxic T lymphocytes, CTLs) and CD4+ helper T (Th) cells, respectively [12-14]. After activation, Th1- and Th2-subtype cells are also able to further activate CTLs by secreting cytokines such as interferons (IFNs) and interleukin (IL)-2 [15]. Then, CTLs and innate immune cells, such as natural killer (NK) cells, natural killer T (NKT) cells and γδ T cells, are recruited to tumor sites to exert antitumor effects [16]. Recently, the significant roles of B cells and follicular helper T (Tfh) cells in this process were reported [17, 18]. One report on CD4+ T cells described antitumor cytotoxicity mediated via cytokines in human bladder cancer [19]. However, the majority of tumor cells exploit immune tolerance instead of being eliminated by immune surveillance [15]. Usually, the condition of the tumor microenvironment (TME) and the infiltration of immune cells determine the survival of malignant cells in tissues and organs [20-24]. Surprisingly, a large number of immune cells do not play a positive role in the TME, but instead, actively participate in cancer immune evasion, resulting in an extremely complicated relationship between cancer and immune cells [25-29]. In addition, the heterogeneity of individual bodies or cells, such as the tumor mutational burden (TMB), metabolic status, microbiome and other specific characteristics, also exert crucial influences on the TME and outcomes of immunotherapy.
Based on current literature, in this review, we discuss the various intracellular and extracellular factors, and regulators associated with cancer and immunity. The latest available technologies and treatment methods for resolving clinical problems in cancer immunotherapy are also discussed, including the controversies and limitations in this field.
2 TYPICAL MOLECULES INVOLVED IN ANTITUMOR IMMUNITY AND THEIR CLINICAL APPLICATION
2.1 Immune checkpoints
2.1.1 Known immune checkpoints
CTLA-4 was the first negative regulator identified to be expressed on T cells. After T cell receptor (TCR) engagement, the expression of CTLA-4 on the T cell surface is upregulated, CTLA-4 is trafficked to the immunologic synapse, and expression finally peaks 2 to 3 days after T cell activation [30, 31]. With a better affinity than the T cell costimulatory molecule CD28, CTLA-4 suppresses T cell function by competitively binding to its ligands CD80 (B7.1) and CD86 (B7.2), which are also the main ligands for CD28 [32, 33]. Therefore, the primary mechanism of CTLA-4 blockade is the release of CD28-mediated positive costimulatory signals such as the phosphoinositide 3-kinase (PI3K) and AKT signaling pathways [34]. In 2011, the Food and Drug Administration (FDA) first approved ipilimumab, a monoclonal antibody (mAb) drug targeting the immune checkpoint molecule CTLA-4, which signaled the beginning of immune checkpoint blockade (ICB) immunotherapy. However, scientists observed that patients with heterozygous germline mutations in CTLA-4 (in human) and Ctla-4 (in animals) knockout mice always exhibited severe immune dysregulation [35, 36], and many oncologists also observed that anti-CTLA-4 monoclonal antibodies (mAbs) frequently induced autoimmune reactions in patients [37]. Further study revealed that this could be attributed to the high expression of CTLA-4 on regulatory T cells (Tregs) [38]. Therefore, there is still a need to investigate how the utility of anti-CTLA-4 therapy could be optimized to maximize its efficacy and minimize associated adverse reactions. Many clinical trials evaluating the therapeutic effect of CTLA-4 blockade alone or in combination with other therapies in many types of cancers, such as advanced renal cell carcinoma, non-small cell lung cancer (NSCLC), and metastatic melanoma, have shown promising results [39-43].
PD-1 is mainly expressed on the surface of activated T cells, and its primary biological function is to form a negative feedback loop to suppress local T cell responses and minimize excessive damage to self-tissues [44, 45]. The main ligands binding to PD-1, programmed death-ligand 1 (PD-L1; CD274) and PD-L2 (CD273), are widely expressed on the surface of nonlymphoid cells such as tumor cells and DCs [46]. When engaged by PD-L1 or PD-L2, PD-1 transduces a negative costimulatory signal to attenuate T cell activation through the tyrosine phosphatase src homology 2 (SH2) domain-containing protein tyrosine phosphatase 2 (SHP-2), which then dephosphorylates CD28 [47]. One study showed that PD-1 engagement could suppress glycolysis and promote fatty acid oxidation and lipid catabolism, which are crucial for T cell functions [48]. Hence, when a T cell is activated, the expression of some inhibitory receptors, such as PD-1, is upregulated to suppress the proliferative capacity and cytotoxic potential, which is defined as T cell exhaustion. Therefore, the primary mechanism of PD-1 signaling axis blockade is to reverse the exhausting function of CTLs [49]. Numerous clinical trials have shown that anti-PD-1 and anti-PD-L1 mAbs are associated with better OS and tolerance than conventional therapies in several types of cancers [50-55]. In 2020, the Nivolumab, Ipilimumab and COX2-inhibition in Early Stage Colon Cancer: an Unbiased Approach for Signals of Sensitivity (NICHE) study showed that 20/20 (100%) patients with microsatellite-instability (MSI) tumors and 4/15 (27%) patients with microsatellite-stable (MSS) tumors had impressive pathological response after receiving ipilimumab combined with nivolumab as neoadjuvant immunotherapy [56]. This was consistent with the results of neoadjuvant ICI treatments in many other cancers [57-59]. Based on findings of these results, ICIs not only yielded promising clinical outcomes in cancer patients with refractory tumors when used as adjuvant therapy but also showed the potential to be used as a new standard therapy in early-stage cancers.
Compared with PD-L2, PD-L1 is expressed more widely in normal and tumor cells, so there are many studies exploring how to disrupt the PD-L1-PD-1 interaction for cancer therapy [60]. The FDA has already approved some mAbs against PD-L1, such as atezolizumab, durvalumab and avelumab, for the treatment of many types of cancer. Furthermore, apart from neutralizing PD-L1 mainly expressed on the tumor cell membrane, many researchers are exploring approaches to reduce the wide expression of PD-L1. Therefore, it is of great necessity to fully understand the upstream regulation of PD-L1, which may aid in the development of new strategies to manipulate the expression of PD-L1 to enhance the response rates of ICI immunotherapy. There are studies that described the PD-L1 regulation, including genomic alterations, epigenetic regulation, posttranscriptional and posttranslational modifications (PTMs), noncoding RNA-based regulation and exosomal transport, and offered novel insights on how to reverse ICI resistance [46, 61, 62]. Moreover, other checkpoints may also be regulated by some unique pathways and molecules, which may be future targets to enhance the antitumor response.
2.1.2 Promising immune checkpoints
It is well known that due to metabolic or hypoxic stress, tumor cells release a large amount of adenosine triphosphate (ATP) into the extracellular space (eATP), and the metabolic product adenosine is able to drive tumor progression by suppressing antitumor immune responses while enhancing the proliferation of immunosuppressive cells [63, 64]. Extracellular adenosine activates G protein-coupled receptors, particularly A2a and A2b receptors, which are mainly expressed on immune cells such as phagocytes, NK cells, and T cells [65], and ultimately suppresses immune responses through the cAMP-protein kinase A (PKA)-mediated inhibition of nuclear factor-κB (NF-kB) and Janus kinase-signal transducer and activator of transcription (JAK-STAT) signaling pathways [66]. Therefore, in recent years, researchers have focused on metabolic pathways of adenosine that can be targeted to suppress tumor development. Once ATP is released into the extracellular space, CD39 hydrolyzes ATP into adenosine monophosphate (AMP), which is further converted into adenosine by CD73 [67]. There are many types of cancers expressing CD73 and CD39, which are both correlated with poor prognosis [68-71]. In 2013, Allard et al. [72] discovered that CD73 blockade had a synergistic effect with anti-PD-1 and anti-CTLA-4 mAbs in mouse models. Additionally, in 2019, Li et al. [64] found that CD39 blockade resulted in accumulation of eATP and increased intratumoral T cell numbers through the eATP-P2×7-ASC-NALP3-inflammasome-IL-18 pathway which could overcome anti-PD1 therapy resistance. The impressive results seen in preclinical experiments have given rise to many clinical trials.
Furthermore, it is also widely known that tumor cells express many “don't eat me” signals to evade clearance by the innate immune system. One typical molecule mediating this phenomenon is CD47, whose expression is upregulated in many types of cancers [73-76]. Signal regulatory protein α (SIRPα) is a specific ligand that binds to CD47 and is mainly expressed on myeloid cells [77, 78]. Upon engagement with CD47, SIRPα recruits inhibitory molecules such as src homology 2 domain-containing protein tyrosine phosphatase 1 (SHP-1) and SHP-2 through its intracellular immunoreceptor tyrosine-based inhibitory motif (ITIM) domain and subsequently suppresses phagocytosis by macrophages and prevents macrophages from clearing tumor cells [79, 80]. Furthermore, CD47 blockade seems to produce a synergistic effect with different antitumor treatments in animal models [80-82]. Additionally, in 2019, Weissman et al. [83] found a completely new “don't eat me” signal, CD24, which could promote immune evasion by interacting with sialic acid-binding Ig-like lectin 10 (SIGLEC-10) and ultimately suppress phagocytosis by macrophages. In 2018, a phase 1b clinical trial showed that the CD47 inhibitor 5F9 combined with rituximab had promising activity in patients with relapsed or refractory lymphoma [84]. In addition, the favorable tolerance of the therapeutic strategy targeting this “don't eat me” signal is being investigated in ongoing clinical trials.
In 2019, Chen et al. [85] found a completely new immune checkpoint, SIGLEC-15, through a genome-scale T cell activity array (TCAA). SIGLEC-15 is mainly expressed on myeloid cells and tumor cells and directly binds to T cells. SIGLEC-15 inhibits antigen-specific T cell responses mainly by regulating cell growth and promoting immune evasion but the specific mechanism underlying these effects is still unclear. More recently, in 2020, Yan et al. [86] discovered that selective inhibition of c-mer proto-oncogene tyrosine kinase (MerTK) on macrophages could also suppress efferocytosis by macrophages to increase the release of ATP and tumor DNA, which activated the cyclic guanosine monophosphate-adenosine monophosphate synthase (cGAS) signaling pathway and enhanced type I IFN-dependent antitumor immune responses. Lu et al. [87] discovered that the depletion of interleukin-1 receptor-like 1 (also known as suppression of tumorigenicity 2, ST2), a receptor of IL-33 that is particularly expressed on tumor-associated macrophages (TAMs), had a synergistic effect with anti-PD-1 treatment in colorectal cancer. Moreover, tumor-secreted granulocyte-macrophage colony-stimulating factor (GM-CSF) further activated c-Rel through the NF-κB signaling pathway in myeloid precursor cells which initiated myeloid-derived suppressor cell (MDSC) differentiation by enhancing the expression of MDSC signature genes such as CCAAT enhancer-binding protein beta (Cebpb) and arginase-1 (Arg1), and eventually inhibited T cell-mediated antitumor responses and promoted tumor progression [88].
Although clinical success has been demonstrated with anti-CTLA-4, anti-PD-1, and anti-PD-L1 mAbs, a large number of patients still do not derive benefit from them. One reason could be that there are many other immune checkpoints, such as lymphocyte activation gene-3 (LAG-3), expressed on the T cell surface, T cell immunoglobulin and mucin domain-containing protein-3 (TIM-3), T cell immunoglobulin and ITIM domain (TIGIT) and V-domain immunoglobulin suppressor of T cell activation (VISTA) [89-92]. The variable interactions among these checkpoints are shown in Figure 1. Although there are many preclinical experiments confirming the antitumor responses induced by targeting these novel immune checkpoint molecules and an increasing number of immune checkpoints are being discovered [93-95], there is still much work that needs to be done to identify additional novel immune checkpoints and develop new therapeutic strategies to target these molecules alone or in combination with other agents to maximize clinical benefits.
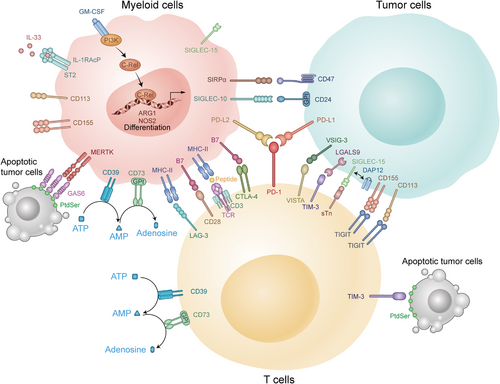
Variable interactions among immune checkpoints in the TME. There are many immune checkpoints in the TME. Some of them are expressed mainly on T cells including PD-1, CTLA-4, and LAG-3, which could suppress the function of CTLs. The others are mainly expressed on myeloid cells, such as c-Rel and MerTK, which could enhance the inhibitory function of MDSCs to tumor cells. Abbreviations: TME: tumor microenvironment; CTLs: cytotoxic T lymphocytes; IL: interleukin; CD: cluster of differentiation; MHC: major histocompatibility complex; PD-1: programmed cell death-1; PD-L1: programmed cell death-Ligand 1; PD-L2: programmed cell death-Ligand 2; CTLA-4: cytotoxic T lymphocyte-associated antigen 4; VISTA: V-domain immunoglobulin suppressor of T cell activation; TIGIT: T cell immunoglobulin and ITIM domain; TIM-3: T cell immunoglobulin and mucin domain-containing protein 3; LAG-3: lymphocyte activation gene-3; ATP: adenosine triphosphate; AMP: adenosine monophosphate; GPI: glycosylphosphatidylinositol; SIRPα: signal regulatory protein α; SIGLEC-15: sialic acid binding Ig-like lectin 15; GM-CSF: granulocyte-macrophage colony stimulating factor; IL-RAcP: interleukin-1 receptor accessory protein; ST2: suppression of tumorigenicity 2; MERTK: c-mer proto-oncogene tyrosine kinase; GAS6: growth arrest specific 6; PtdSer: phosphatidylserine; TCR: T cell receptor; SIGLEC-10: sialic acid binding Ig-like lectin 10; VSIG-3: V-set and immunoglobulin domain-containing protein 3; LGALS9: galectin-9; DAP12: DNAX-activation protein 12; sTn: sialyl-Tn; PI3K: phosphoinositide 3-kinase; ARG1: arginase-1; NOS2: nitric oxide synthase 2
2.2 Soluble cytokines
More than 30 years ago, scientists purified the protein IL-2, and found that it plays an important role in activating T cell growth [96]. It is mainly produced by activated T cells. IL-2 was approved for the treatment of metastatic renal cell carcinoma by the FDA and has shown promising results as a single agent [97]. However, in addition to stimulating T cell proliferation and memory T cell differentiation, IL-2 also promotes the generation and function of Tregs [98-100] which has been associated with many side effects and has limited its further use [97]. Therefore, the most sensible next step was to establishing clinical trials to evaluate the effect of IL-2 in combination with other drugs, including IFN-α, cisplatin, dacarbazine, and adoptive cell therapies (ACTs) [101-104]. However, a phase I/II study showed that the objective response rate of IL-2 in combination with CTLA-4 blockade was not better than that of IL-2 alone [105]. Therefore, whether IL-2 can enhance the efficacy of immunotherapies may require more experimental and clinical investigations.
In addition to IL-2, IFN-γ plays a pivotal role in the development of tumors. The biological functions of IFN-γ are mediated mainly through the JAK-STAT signaling pathway which regulates the transcription of many genes [106]. However, the functions of IFN-γ are contradictory because this cytokine can also suppress tumors in multiple ways. For example, IFN-γ suppresses tumor cell proliferation through cyclin-dependent kinase inhibitor 1 (p21) [107] and promotes tumor cell apoptosis through the upregulation of caspase, Fas and Fas ligand expression [108, 109]. IFN-γ also promotes the recruitment of CTLs through a variety of chemokines such as CXCL9, CXCL10, and CXCL11 [110]. IFN-γ was approved for the treatment of severe malignant osteopetrosis in 2000 [111]. An IFN-related gene signature can be used to predict the efficacy of different therapies, including immunotherapy, in many types of malignancies [112-114]. There are many studies showing that ICI treatment can improve the effects of targeted therapy and radiation in an IFN-γ-dependent manner [115, 116]. In addition, tumors produce high levels of IFN-γ after ICI treatment [117, 118]. On the other hand, IFN-γ is capable of supporting tumorigenesis. IFN-γ can induce tumor cells and TAMs to express many inhibitory receptors such as PD-L1 and PD-L2 [119]. IFN-γ can also upregulate the expression of indoleamine-pyrrole 2,3-dioxygenase (IDO) in melanoma cells, which recruits Tregs to avoid immune attack [120]. Therefore, IFN-γ has not shown significant clinical benefit in many types of cancers such as metastatic renal cell carcinoma, breast cancer, and colon cancer [121-123]. There are some reasons that partially account for this result; for instance, IFN-γ is rapidly cleared after intravenous administration [124], so it cannot be delivered locally at a sufficient concentration to achieve a therapeutic effect [111]. As such, the functions of IFN-γ are quite complex, and the efficacy and tolerance of IFN-γ-based therapies require further investigation.
There have been studies confirming that some other T cell growth factors, such as IL-15, IL-7, IL-12, and IL-21, also have antitumor potential through modulation of T cell expansion, survival and functions [125-128], and may have synergistic effects with ICIs or other immunotherapies [129]. However, many soluble cytokines are negatively correlated with the clinical benefit derived from ICI immunotherapy. For example, many researchers observed that elevated serum IL-8 levels were associated with increased intratumoral neutrophil levels and a relatively poor prognosis for ICI immunotherapy [130, 131]. TGF-β also suppresses antitumor immune responses in the process of cancer progression [132]. Thus, the application of these soluble cytokines seems promising, and more in-depth research must be conducted.
Taken together, among the molecules mentioned above, the applications of PD-1, PD-L1, PD-L2, and CTLA-4 are the most widespread. Drugs targeting other molecules are still under development. Whether they could be applied in the clinic remains unclear. Therefore, further research should be performed to utilize these targets for immunotherapy. We expect to obtain more effective drugs for patients through different clinical trials.
3 FUNDAMENTAL MODULATORS OF ANTITUMOR IMMUNITY
3.1 Genome, epigenetic regulators, and posttranscriptional modulation
There are a variety of intracellular and extracellular factors that influence the efficiency of cancer immunotherapy. The genome and mutations in the chromatin of tumor cells should be mentioned first because genes determine the phenotype of a single cell and the basic immune response. For example, Asian patients with NSCLC may obtain greater benefit from atezolizumab in terms of OS than Caucasian patients [133], and male patients with melanoma show higher TMB and PD-L1 expression than female patients, while female patients with lung squamous cell carcinoma (LUSC) have a higher T cell-inflamed gene expression profile (GEP) and cytolytic activity (CYT) than males [134].
Similar to normal cells, tumor cells contain HLA genes that encode MHC protein expression on the cell surface. Then, antigens specific to tumor cells can be recognized by immune cells mediating an immune response. Thorsson et al. [135] summarized 6 immune subtypes of cancer-based on over 10,000 samples from 33 different tumor types in The Cancer Genome Atlas (TCGA): TGF-β Dominant, Wound Healing, Lymphocyte Depleted, IFN-γ Dominant, Immunologically Quiet, and Inflammatory. In addition, they listed some specific driver mutations associated with leukocyte levels, including mutations in TP53, BRAF, CASP8, NRAS, CTNNB1, and IDH1. The TMB has already been recognized as a favorable prognostic marker for immunotherapy in different kinds of tumors [136-138]. Nonsynonymous single-nucleotide variants (nsSNVs) are the main sources of the TMB and serve as immunogenic peptides for immune responses [139]. In 2020, the FDA identified high TMB (TMB-H) as an indication for PD-1 inhibitor Keytruda use, regardless of the tumor type. Furthermore, deficiency in mismatch repair (MRR) genes with high microsatellite instability (MSI-H) is a reason for an increased TMB, especially in colorectal cancer (CRC). In 2017, two ICIs (pembrolizumab and nivolumab) were first approved by the FDA for the treatment of patients with MMR-deficient (dMMR) CRC [140]. Unfortunately, almost 85% of CRC cases are classified as MMR proficient (pMMR), representing a status of MSS or low microsatellite instability (MSI-L) [141]. For patients with pMMR CRC, many immunotherapy clinical trials have failed to demonstrate a benefit, probably due to low infiltration of immune cells [142-144]. Emerging neoantigens and mutations that promote oncogenic outgrowth could be targets of the immune system and limit the spread of malignancies [145]. In contrast, tumor cell death following immune attack or other intrinsic stresses may also result in the release of damage-associated molecular patterns (DAMPs), such as extracellular ATP, high mobility group box 1 (HMGB1) and adenosine, causing escape from immune surveillance [146, 147].
During the transcription and translation of antigenic proteins, epigenetic and posttranscriptional modulators also exert a significant influence on antigenic protein expression. DNA or histone methylation is the most common mechanism of epigenetic regulation in cells. For instance, excessive methylation of the MMR gene MLH1 represses its expression and leads to the accumulation of cellular mutations in CRC [148, 149]. In addition to cancer cells, immune cells also exhibit a reprogrammed epigenome which is a common factor associated with prognostic value [150]. Chronic viral infections have been reported to induce T cell exhaustion via de novo methylation [151]. For posttranscriptional modulation, ubiquitination is a representative and useful process to manipulate immune responses. Diverse E3 ubiquitin ligases mediate the degradation of some key proteins in various cancer cells and immune cells, affecting their interaction, such as destabilizing Foxp3 to attenuate Treg functions [152, 153]. In addition, other regulatory networks, such as histone acetylation, noncoding RNAs and autophagic degradation, have shown unique roles with therapeutic potential in antitumor immunity [154-157]. Currently, many epigenetic therapeutics targeting DNA, including methyltransferase inhibitors and histone deacetylase inhibitors, have been widely used in clinical trials, and some of them are being tested in combination with ICIs [158]. Notably, the application of such inhibitors, such as hypomethylating agents (HMAs), may also induce immunosuppression [159] and which warrants caution.
3.2 Metabolism and diet
Metabolism comprises a very large system that maintains the stability of cells and promotes their adaptation to changes of the external environment. Tumor and immune cells have many metabolic similarities and differences. Most cells tend to generate ATP through oxidative phosphorylation (OXPHOS) instead of aerobic glycolysis when oxygen resources are abundant. In contrast, activated glycolysis is the preferred glucose metabolism pathway in malignant cells [160, 161]. For T cells, there is a shift in dominance between glycolysis and OXPHOS during cellular differentiation [162, 163]. Naïve T cells and memory T cells depend more on OXPHOS than glycolysis, while effector T cells metabolize more glucose through glycolysis [164, 165]. Lipid accumulation is another crucial profile in the metabolic reprogramming of the TME [166, 167]. Long-chain fatty acids accumulate in T cells, restricting their mitochondrial function [168]. As an important process for tumor survival, fatty acid oxidation (FAO) is usually suppressed in effector T cells but is increased in naïve T cells and memory T cells [165, 169]. On the other hand, both OXPHOS and FAO are critical for the function of Tregs [170]. In addition, T cells take up plentiful glutamine, one of the most abundant amino acids in the body, which is similar to the ‘glutamine traps’ in tumors [163]. Therefore, the metabolic process overlaps between cancer cells and immune cells causing a ‘nutrition battle’ between them based on the basic metabolic status of the patient (Figure 2). For example, accelerated glycolysis in tumor cells may cause glucose deprivation in T cells, attenuating their activity [171, 172]. Moreover, there are also competitions for nutrients, such as arginine, and alternative energy sources, such as inosine, among immune cells, making the interplay in the TME much more complicated [173, 174]. These metabolic overlaps and competitions challenge the traditional view that we can kill cancer cells in vivo by editing their metabolic processes, as this approach may also impair the effects of antitumor immunity. Thus, appropriate metabolic regulation of the TME is needed to enhance immunotherapy. Selective inhibition of some metabolically redundant pathways may be a good option for treatment [175].
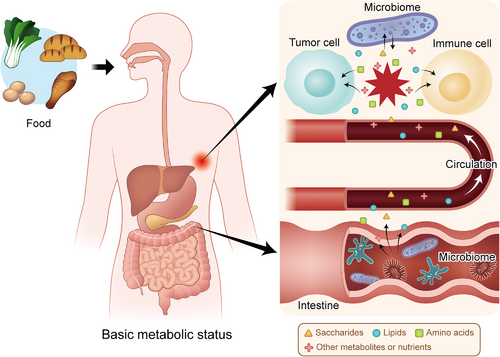
Metabolic interactions in the TME are based on the basic status of the patient. Food is digested and decomposed into metabolites and nutrients based on the basic metabolic status of the human body. The intestinal microbiome participates in the metabolism of these small molecules and influences their levels in the blood. Then, the metabolites and nutrients are sent to the tumor site, forming a competition for nutrients between tumor cells and immune cells in the TME, which is also affected by the local microbiome. Abbreviations: TME: tumor microenvironment
Among different metabolic diseases and disorders, obesity is the most striking global health problem and is correlated with many types of cancers [176]. In addition, obesity not only affects the anabolism or catabolism of fat but also disturbs the transition processes associated with other energy elements. For example, obesity-related insulin resistance increases blood glucose levels, disturbing macrophage phagocytosis [165, 177, 178]. Obesity also causes reduced glycolysis and increased FAO in effector CD8+ T cells, which negatively impacts their functions [179]. However, there are also some contradictory findings associated with obesity [180]. For instance, obesity leads to elevated levels of pro-inflammatory adipokine leptin, which enhances the activity of both CD8+ T cells and CD4+ T cells [181-183]. However, leptin also causes increased PD-1 expression and CD8+ T cell functional exhaustion, attenuating antitumor immunity [180, 184]. Currently, important questions regarding the association between obesity and immunity remain unanswered, and what is already discovered may only be the beginning.
Dietary adjustment may be a good solution for people with obesity or metabolic syndrome. Previous studies have confirmed that dieting improves the effects of chemotherapy [185-187]. Moreover, fasting is also linked to elevated adiponectin levels and reduced glucose, insulin, and leptin levels following the downregulation of cAMP- PKA and insulin-like growth factor 1 receptor (IGF1R)-AKT-mechanistic target of rapamycin kinase (mTOR)-S6K signaling [188]. Fasting-mimicking diets can reduce HO1 levels, leading to an increase in CD8+ tumor-infiltrating lymphocytes (TILs) and a reduction in Tregs [189]. The interaction between the diet and immune system is extremely complicated as patients may need a larger energy supply during treatment. Therefore, much work is probably needed before doctors can create a proper food management system for patients with cancer.
3.3 Microbiome
Apart from creating direct metabolic alterations, changes to our diet may also influence the microscopic organisms that live within us [190] (Figure 2). The gut microbiome is composed of bacteria, viruses, fungi and some special microbiomes, such as archaea [191]. The influences of these commensals on carcinogenesis, metastasis and the effects of conventional therapeutics are broadly recognized [192-195], and many studies have also emphasized their impacts on immunotherapy [196-198]. In 2015, two teams of researchers identified positive correlations between the flora Bacteroides and Bifidobacterium species in the intestinal tract of mice and the effects of ICIs [199, 200]. Subsequently, several intestinal microbe families have been discovered to have specific influences on immunotherapy. The intestinal microbiota is not only indispensable for maintaining the structure and functions of the mucous layer but also interacts with some immune cells, such as Th17 cells, Tregs and DCs. Recently, Zhang et al. [201] found that MDSCs were selectively recruited by Fusobacterium nucleatum, impairing the immune response of the host against CRC. In addition, some gut commensals are deeply involved in the metabolism of certain nutrients or act as facilitators of nutrients such as bile acids (BAs) to modulate inflammatory signaling in immune cells and the proportions of some T cell subtypes [202, 203]. Therefore, dysbiosis is deeply associated with the affected response and toxicity of antitumor immunotherapy [192]. As a consequence, there have been reports of antibiotics negatively impacting the outcomes of patients undergoing immunotherapy [198, 204]. In contrast, fecal microbiota transplantation (FMT) and administration of prebiotics or probiotics have been shown to have great therapeutic potential in animal experiments and clinical trials [200, 205, 206].
Unlike the well-known intestinal commensals, the microbiome within tumor tissues has not attracted enough attention even though many studies have indicated that some of the bacteria existing inside tumor cells have the potential to affect therapeutic effects [207-210]. To obtain a clear understanding of intracellular bacteria, Nejman et al. [211] comprehensively analyzed 7 types of tumors (melanoma, bone, brain, lung, breast, ovarian, and pancreatic tumors) compared with adjacent normal tissues. They found that the content of bacteria in these tumors varied from 14.3% for melanoma to 62.7% for breast tumors. Even some solid tumors without direct contact with the external environment, such as glioblastoma multiforme (GBM), possess live microbes in both cancer and immune cells (Figure 2). Additionally, different kinds of cancer exhibited distinct colonies of bacteria associated with specific metabolic signaling pathways. For example, some bacteria with the capacity to degrade cigarette smoke metabolites were more likely to be enriched in the NSCLC cells of smokers than in those of never-smokers. Moreover, melanoma with a relatively large proportion of Clostridium showed higher sensitivity to immunotherapy, while Gardnerella vaginalis was associated with a poor response to ICIs in patients with melanoma. Thus, the intracellular microbiota plays critical roles in tumor development and the immune response, and more investigations on its biological process are expected to be performed.
3.4 Immune contexture
The immune contexture is a concept similar to the TME that emphasizes the density, distribution and functional interactions among different cells, including but not limited to tumor cells (especially in solid malignancies), immune cells, stromal cells and vessel cells. This concept can be traced back to 2007, when Jérôme Galon, Wolf-Herman Fridman and Franck Pagès elucidated the adaptive immune microenvironment in CRC [212]. Their team summarized three major types of immune contextures, ‘hot’, ‘altered’ and ‘cold’, based on immunoscores evaluating the infiltration of CD3+ and CD8+ lymphocytes [213]. The ‘altered’ phenotype with an intermediate immunoscore was further divided into two categories (altered-excluded and altered-immunosuppressed) to form a more comprehensive classification together with hot and cold phenotypes [213, 214]. Hot or inflamed tumors are infiltrated by abundant TILs, so they show considerable response to ICIs, while cold or noninflamed tumors exhibit the opposite condition with a low TMB and the absence of antigen presentation. Altered-excluded tumors contain TILs mainly at the margin with a reprogrammed TME while altered-immunosuppressed tumors allow only a few TILs to infiltrate without further expansion or recruitment but possess a relatively high proportion of immunosuppressive cells and molecules. Moreover, the formation of the immune contexture depends on some important cytokines or chemokines, such as IFN-γ, perforin and IL-15 [215, 216], key enzymes or factors, such as vascular endothelial growth factor (VEGF) and IDO1 [217, 218], and diverse pathways, including the NF-κB, STAT3 and WNT-β-catenin pathways [219-221]. In 2016, Fridman et al. [222] proposed another classification system based on the infiltration and tertiary lymphoid structures (TLSs) of immune cells as well as vascularization, namely, ‘immunogenic’ (with good prognosis), ‘inflammatory’ (with poor prognosis) and ‘immune neglected’ (with intermediate prognosis). Regardless of how the categories were partitioned, the identification of these profiles was meant to predict the baseline antitumor immunity from a complex TME. Therefore, turning cold or altered tumors into hot tumors with a better immune response could be one of the most pivotal treatment strategies in the future.
Cancer and immune cells interact with each other in a dynamic manner. Thus, we should consider the immune contexture as an adaptive and adjustable system. One noteworthy phenomenon is T cell exhaustion, which usually arises from chronic infection or continuous exposure of T cells to antigens [223, 224]. Viral or bacterial infection and local inflammation can lead to ongoing TCR activation along with sustained expression of inhibitory receptors, such as PD-1, LAG-3 and TIM-3, limiting chronic stimulation [225-228]. This process affects both CD4+ and CD8+ T cells by dramatically reducing their abilities to proliferate and generate cytokines [229, 230]. In contrast, immunosuppressive cells such as Tregs and MDSCs may be increased and aggravate the dysfunction of effector and memory CD8+ T cells by releasing immunosuppressive cytokines [224, 231, 232]. Furthermore, exhausted T cells may express some exclusive molecules, including T-bet and eomesodermin (EOMES) [233], so identifying exhausted T cells with these markers can help facilitate a more precise understanding of the immune contexture.
There are also other aspects of the immune contexture that should be emphasized. For example, sufficient infiltration of some immune cells, such as TAMs and MDSCs, is tightly linked with immune tolerance and cancer progression [234-236]. Another important factor is the effect of tissue-specific immunity during immunotherapy treatment, since different organs exhibit distinct immunological characteristics, the TME of the primary lesion is different from that in metastases and thus influences the efficiency of immunotherapy in tumors in different organs [237]. In summary, we must assess the immune contexture from a dynamic perspective to guide and adjust the immunotherapy regimen.
Take together, the immune response of tumors is a dynamic process that is not only based on the genome, epigenetic regulators and posttranscriptional modulation but also changes with the evolving metabolism and immune contexture. Adjustment of diet and the microbiome in the body could exert a great influence on the TME and the outcomes of cancer immunotherapy.
4 ADVANCED CLINICAL ASSESSMENT AND TREATMENT TECHNOLOGY
4.1 Predicting the clinical outcome of immunotherapy
As mentioned above, the emergence of ICIs has revolutionized the treatment of many types of tumors. However, although clinical benefit has been observed in numerous patients, there are still many patients who do not respond to currently available immunotherapies. Therefore, it is of great importance to distinguish patients who will respond to immunotherapy to maximize the cost-benefit ratio.
4.1.1 Surgical or tissue biopsy specimens
First, multiplex immunohistochemistry (mIHC) is commonly used to assess tissue pathology [238]. As described above, the infiltration of immune cells into the TME can directly influence antitumor effects. Therefore, TILs have been regarded as a significant index correlated with the outcomes of immunotherapy [239-241]. Moreover, the most direct approach to distinguish whether a patient can respond to ICI immunotherapy is to detect whether the targeted immune checkpoint molecule is expressed in tumor specimens. Compared with patients with lower PD-L1 expression levels, those whose tumors were ≥50% PD-L1+ have been shown to have a higher response rate to anti-PD-1 mAbs and better survival [242]. Based on this result, the assessment of PD-L1 expression in tissue specimens by mIHC was approved by the FDA as a companion diagnostic test for pembrolizumab treatment in advanced NSCLC in October 2015 [243]. The immunohistochemical detection of other immune checkpoint molecules, including CD73, LAG-3 and TIM-3, in tissue sections has also been widely investigated [69, 244].
In addition, as sequencing technology gradually matures, there is an increasing number of next-generation sequencing (NGS)-based assays to identify critical somatic mutations that can predict response to immunotherapy. For example, in 2017, one meta-analysis including 27 tumor types or subtypes revealed that the TMB had a significantly positive correlation with the objective response rate in patients treated with anti-PD-1 or anti-PD-L1 therapy [245]. In 2019, a combined analysis including 47,721 patients found POLE/POLD1 mutations to be independent predictive biomarkers for a positive clinical benefit in many types of cancer [246]. These results suggest that genome sequencing data for tumor specimens can play a critical role in guiding the selection of patients who will respond to immunotherapy.
Finally, although great progress has been made in other traditional sequencing technologies, including DNA sequencing, RNA sequencing and protein sequencing, regarding precision and throughput of diagnosis, these technologies are based on bulk cell populations and provide information that is a steady-state average of thousands of cells. However, the heterogeneity of a cell population, which is mainly governed by genetic heterogeneity, epigenetic and developmental programs, and extrinsic and spatial factors, may determine many essential complicated biological behaviors such as tumor growth, metastasis, and treatment resistance [247], which traditional sequencing technology can overlook. Single-cell sequencing can overcome these shortcomings as it examines the sequence information of individual cells and provides a better understanding of the heterogeneity and evolutionary relationships of various cells in a microenvironment. Therefore, it is widely utilized in tumor research [248, 249]. In 2019, Suvà and Tirosh [247] reviewed the common themes, opportunities and challenges, and emerging single-cell genomic methods of single-cell sequencing, especially single-cell RNA sequencing in cancer, and then provided new methods and ideas for the investigation of cancer biology. In 2020, Regev et al. [250] introduced the concept of a dynamic three-dimensional (3D) atlas of cancer transitions for a diverse set of precancerous lesions and established tumors at single-cell resolution, called the Human Tumor Atlas Network (HTAN), and is planned to be finished within the next five years. Once completed, this work could greatly improve the understanding, diagnosis, monitoring, drug development, and biomarker discovery of tumors, which could eventually boost developments in precision medicine.
4.1.2 Liquid biopsy
In clinical diagnosis, tumor tissue biopsy is regarded as the standard of choice to investigate whether a patient will respond to immunotherapy [251]. Liquid biopsy is a newly developed technique that can detect the genomic profile of patients with cancer utilizing bodily fluids such as blood, urine, saliva, pleural effusion, and cerebrospinal fluid. Circulating cell-free DNA (cfDNA), circulating tumor DNA (ctDNA), circulating tumor cells (CTCs), proteins, exosomes and other circulating components can all be analyzed through liquid biopsy [252]. Compared with traditional tissue biopsy, liquid biopsy is less invasive and more cost-effective, accessible, and replicable [251]. Subsequently, there are also several studies exploiting the application of liquid biopsy in cancer management [253-256].
For immunotherapy, liquid biopsy also exhibits unique potential to predict the prognosis of patients with cancer. In 2015, Mazel et al. [257] first detected PD-L1 expression on CTCs in patients with breast tumors and created a new method to monitor PD-L1 expression in solid tumors. Later, in 2018, Chen et al. [258] discovered that PD-L1 expression on circulating exosomes before or during anti-PD-1 treatment reflected the different states of antitumor immunity. In addition, a proof-of-principle study revealed that the detection of ctDNA 8 weeks after anti-PD-1 treatment was negatively correlated with survival in patients with NSCLC, uveal melanoma, or MSI CRC [259]. Gandara et al. [260] demonstrated that the TMB in the blood (bTMB) could be a promising biomarker to predict clinical benefit from ICI immunotherapy. The inconsistency in results from previous studies may be partly due to sample instability, immature quantification and isolation methods, low sensitivity, and high cost [252, 261]. liquid biopsy has the potential to exhibit a correlation with the clinical outcomes of immunotherapy and improve clinical diagnosis.
4.2 Advanced treatment technology
With the rapid development of oncoimmunology, immunotherapy has been approved for the treatment of multiple cancers and has achieved surprising clinical outcomes in recent decades. Nevertheless, we still face great challenges in translating experimental observations into clinical practice. First, as mentioned above, not all patients respond to ICIs. Second, the main approach to delivering immunotherapy is through systematic administration, which may result in many immune-related adverse events (irAEs) because of off-target effects and the fact that many drugs cannot reach solid tumors at sufficient levels, especially when facing many delivery barriers [262, 263]. Therefore, developing other advanced treatment technologies to improve the safety and efficacy of immunotherapy is urgently needed. Some of these novel technologies are described below.
4.2.1 Cell-based immunotherapies
T cells are the major type of cells that directly kill tumor cells in the TME. Cell-based immunotherapy is mainly referred to as chimeric antigen receptor (CAR)-T cell immunotherapy, in which doctors collect T cells from the patient (autologous) or a healthy person (allogenic), genetically engineer the cells to express an artificial CAR specifically targeting an antigen presented on tumor cells, and then administer the cells in patient to eradicate tumor cells [264]. The first experimental target was CD19, which is mainly expressed on B cell leukemia and lymphomas. The first patient treated with anti-CD19 CAR-T cells achieved promising outcome, and this success encouraged a series of studies [265]. The FDA approved anti-CD19 CAR-T cells for the treatment of refractory pre-B cell acute lymphoblastic leukemia and diffuse large B cell lymphoma in 2017, which revolutionized cancer immunotherapy [266]. Moreover, the application of CAR-T cells is not limited to only CD19 and it has been extended to CD22 and BCMA [267, 268].
However, there are still many challenges for this therapy. First, cytokine release syndrome is frequently observed during treatment [269]. Second, although CAR-T cell therapy has achieved excellent results in hematological tumors, it has not been as effective in solid tumors [270, 271]. To overcome this limitation, one study tried to use bioengineered polymer scaffolds, which when implanted near or at the resection sites of tumors were able to stimulate and expand tumor-reactive T cells, producing a curative effect in mouse models of solid tumors [272]. In addition, scientists are also investigating several novel methods such as synthetic APCs and nanoparticle-functionalized T cells [273, 274]. Nevertheless, more evidence is required to show whether these methods can be further developed and introduced into clinical trials.
Although CAR-T cell immunotherapy has exhibited impressive results in hematological malignancies, it usually does not provide enough value in solid tumors [275]. In contrast, TCR-engineered T (TCR-T) cells may show greater promise in solid tumors because engineered TCRs consist of a glycoprotein alpha-beta chain heterodimer that can bind to peptides presented by MHC molecules [276]. As a result, TCR-T cells can recognize both surface proteins and intracellular proteins, targeting many more antigens and penetrating tumors better than CAR-T cells [277]. Most of the clinical trials on TCR-T cells are still in phase 1 or phase 2, and the cost and time-consuming process of TCR cloning limit the broad application of TCR-T cell therapy [276]. Furthermore, several researchers believe that a combination of CAR-T and TCR-T cell immunotherapies will have therapeutic effects on solid tumors in the future because their mechanisms of action and resistance are completely different from those of traditional CAR-T cell immunotherapy.
Similarly, to overcome the challenge of solid tumors not satisfactorily responding to CAR-T cell immunotherapy, Gill et al. [278] focused on macrophages, which are very abundant in the TME of many types of solid tumors. The authors produced chimeric antigen receptor macrophages (CAR-Ms) that secreted proinflammatory cytokines and upregulated antigen presentation, ultimately enhancing their antitumor ability. In humanized mouse models, CAR-M therapy significantly diminished the TMB and prolonged OS [278]. However, there are still many limitations in CAR-M therapy. For example, CAR-Ms could not proliferate in vitro or in vivo, and the biodistribution of CAR-Ms after systemic administration also largely influenced the response rate. Maybe we could combine CAR-Ms with some other treatments such as cytokine therapy or nanoparticle-based approach to overcome these shortcomings.
DCs are the most powerful APCs in the human body and have garnered considerable attention in the development of novel therapeutic cancer vaccines. The manufacture of DC vaccines generally starts with the isolation of autologous DCs, followed by exposing them to an appropriate source of tumor-associated antigens (TAAs) ex vivo and then, refusing them back into the patient. A series of clinical trials have already been undertaken in patients representing many types of cancers, which demonstrated the safety and therapeutic profile of DC vaccines [279]. However, DC vaccination may have limitations as monotherapy because of the immunosuppressive microenvironment, so the combination of DC vaccination with other therapies, such as ICB, chemotherapy, and radiation therapy, may enhance antigen-specific antitumor immunity [280].
4.2.2 Oncolytic virus immunotherapies
Scientists have invented various vaccines against viruses to prevent oncogenesis [281], but they are also currently using viruses as immunotherapy. As described above, ‘cold tumors’ usually do not respond well to immunotherapy. To overcome resistance to ICIs, there are many combination therapies under investigation, including approaches turning ‘cold tumors’ into ‘hot tumors’ [282]. Due to their abilities to infect tumor cells and propagate within these cells, oncolytic viruses (OVs) can selectively kill cancer cells, resulting in the release of TAAs, additional DAMPs, viral pathogen-associated molecular patterns (PAMPs), and other molecules, such as cytokines, to induce an antitumor immune response [283]. The first approved OV therapy was talimogene laherparepvec (T-VEC), which is based on a type 1 herpes simplex virus (HSV-1). With the deletion of ICP34.5 and ICP47, T-VEC can specifically replicate in and lyse tumor cells, ultimately inducing local and global antitumor immunity [284]. A phase III clinical trial investigating the efficacy and tolerance of T-VEC successfully treated advanced-stage melanoma [285]. However, this current OV therapy works only in a few select types of tumors. Although there are many novel methods of drug delivery under investigation, the dose-effect relationship of OVs cannot be predicted easily due to their self-replication [284]. Moreover, the application of OV therapy in combination with other immunotherapies remains in the experimental stage.
4.2.3 Neoantigen vaccines
In addition to OV administration, increasing the expression of neoantigens is another way to turn ‘cold tumors’ into ‘hot tumors’ which could induce specific antitumor immune responses. In 2017, two successful cases of personalized neoantigen-based tumor vaccines for the treatment of advanced melanoma were published in Nature at the same time [129, 286]. One report was about an RNA-based polypeptide vaccine that produced sustained progression-free survival (PFS) in over 60% (8/13) of patients [286]. The other was about a vaccine that targets up to 20 predicted personal tumor neoantigens which led to no recurrence in nearly 70% (4/6) of patients for 25 months [129]. Although the sample numbers were relatively limited, the results are extremely inspiring. Later, in 2020, one team generated personalized neoantigen vaccines based on NGS with their in-house pipeline iNeo-Suite. This was the first pan-cancer clinical study concentrating on personalized neoantigen vaccine monotherapy that showed promising feasibility, safety, and efficacy [287].
4.2.4 Nanoparticle-based approaches
To date, a diversity of immunotherapies, including ICIs, ACTs, tumor vaccines, OVs and cytokine therapies, have been established. The effects of these therapies rely largely on their interactions with targeted molecules or cells, so an efficient delivery technology could strikingly improve the effect and safety of these therapies [262]. A typical example of nanoparticle-based approaches is nanoparticle-programmed CAR-T cells [288]. Nanoparticles that encapsulate tumor CAR-encoding DNA recognize circulating T cells through CD3 molecules in the blood, releasing the DNA into the T cells to achieve sufficient cellular CAR expression to eradicate tumor cells. This method has achieved great success in mouse models of B cell lymphoblastic leukemia, which provided a novel idea for making CAR-T cell therapy possible in hospitals without the need to engineer T cells ex vivo in a special laboratory. Moreover, another study used nanoparticles to deliver tumoral mRNA in vivo [289]. Researchers have used lipid-based nanoparticles to package mRNA transcripts encoding tumor neoantigens. Systemic administration into multiple mouse models of established tumors then led to the expression of TAAs by local APCs and subsequently induced durable type I IFN-dependent antigen-specific immunity. In summary, nanotechnology can improve the safety and efficacy of immunotherapy by better controlling the dose, location, release, and penetration of immunotherapeutic drugs or cells as well as by optimizing the treatment process. As a consequence, nanotechnology could make tumor immunotherapy more comprehensive and more effective. Despite these achievements, more research and clinical trials on nanoparticles are needed to further confirm their therapeutic effects.
Currently, the application of these new technologies is not as universal as ICIs, and some of them are still under preclinical study. Nevertheless, they could enhance the immunological function of our body through different mechanisms, and they are powerful complements for existing immunotherapies. We believe that if we could utilize them more reasonably on basis of thorough exploration, a new chapter on immunotherapy could be started in the near future.
5 CONTROVERSIES AND LIMITATIONS OF CANCER IMMUNOTHERAPY
5.1 Adverse effects of cancer immunotherapy
For over a century, until the development of anti-PD therapy (anti-PD-1 and anti-PD-L1 antibodies), cancer immunotherapy was not popular. Despite great efforts being made in the investigation of cancer immunotherapy, low efficacy and frequent irAEs have limited its clinical application.
Before anti-PD therapy, only five types of cancer immunotherapy were approved for clinical use, i.e., IFN (for hairy cell leukemia, kidney cancer, and melanoma) [290-292], IL-2 (for kidney cancer and melanoma) [293, 294], sipuleucel-T (a cancer vaccine for prostate cancer) [295], CAR-T cells (for B cell lymphoma/leukemia) [296], and anti-CTLA-4 mAbs (for melanoma) [297]. Most of the above therapies resulted in frequent irAEs and low objective response rates, which dramatically limited their use to only several cancer types. For example, although only a small proportion of melanoma patients respond to anti-CTLA-4 mAbs (14% on average), grade 3-4 AEs are common (27%) [38]. Similarly, IL-2 can induce a general capillary leak syndrome that severely affects multiple organs [298], resulting in frequent grade 3-4 AEs (>43%) [38].
In contrast, newly developed anti-PD therapies have a higher objective response rate with many fewer irAEs (Figure 3). For example, CheckMate 067, a phase III clinical trial comparing nivolumab (an anti-PD-1 antibody) and ipilimumab (an anti-CTLA-4 antibody) in previously untreated patients with metastatic melanoma, showed that nivolumab produced more than two times the number of antitumor responses than ipilimumab (43.7% versus 19.0%), while the number of grade 3-4 treatment-related AEs was lower with nivolumab than with ipilimumab (16.3% versus 27.3%) [299]. A systematic review involving 20,128 patients showed that the severe toxicity (grades 3-5) rate of anti-PD therapy, including anti-PD-1 and anti-PD-L1 antibodies, was only 14.0%. The most common all-grade AEs were fatigue (18.26%), pruritus (10.61%), and diarrhea (9.47%). The most common grade 3 or higher AEs were fatigue (0.89%), anemia (0.78%), and an increased aspartate aminotransferase level (0.75%). Hypothyroidism (6.07%) and hyperthyroidism (2.82%) were the most frequent all-grade endocrine irAEs [300]. The safety profile of anti-PD therapy is also better than that of conventional chemotherapy. A meta-analysis involving 22 randomized immunotherapy trials, 21 of which were anti-PD therapy trials, versus standard-of-care chemotherapy trials showed that immunotherapy was associated with a significant reduction in the likelihood of developing severe AEs (16.56% versus 41.09%) and AEs of any grade (65.82% versus 85.19%) [301].
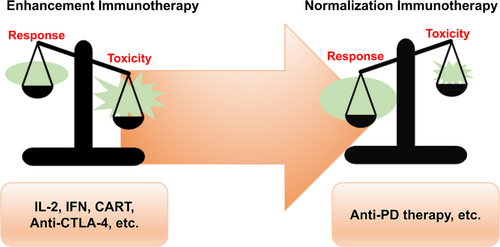
The balance between response and toxicity in anti-PD therapy compared with other immunotherapy methods. Toxicity outweighs the response in immunotherapy methods such as IL-2, IFNs, CAR-T cells, and anti-CTLA-4 antibodies, resulting in quite limited indications. For anti-PD therapy, the response outweighs toxicity considerably, which leads to a rather broad application. Abbreviations: CAR-T: chimeric antigen receptor T cells; CTLA-4: cytotoxic T lymphocyte-associated antigen 4; IFN: interferon; IL-2: interleukin-2; PD: programmed death
Despite the improved safety profile, greater attention should be given to the special irAEs of anti-PD therapy, which can be fatal. IrAEs most commonly affect the gastrointestinal tract, endocrine glands, skin, and liver [302], but severe pneumonitis, myocarditis, colitis, hepatitis and neurological toxicities can also occur and lead to a dismal prognosis [303-307]. For instance, fulminant ICI-related myocarditis has been reported to increase in occurrence with the increased use of ICIs [304]. A retrospective analysis of 101 severe myocarditis cases following the administration of ICIs, where the majority of the patients were treated with anti-PD monotherapy or combination therapy, showed that death occurred in 46% of these patients [308]. Therefore, the precise administration of anti-PD therapy and early recognition of severe irAEs are of great clinical significance.
Hitherto, most irAEs can be observed and reported only in clinical trials and real-world studies, and the study of irAEs in preclinical and translational investigations is still limited [309]. Early data suggest that ICIs can cause malfunctions in the self-tolerance system: the diversification and subcompartmental expansion of lymphocytes activated by ICIs probably gives rise to autoreactive T cells and B cells, causing autoimmune responses [310-312]. In addition, after the activation of immune cells, normal cells distributed in or near the TME may also be damaged, which can result in the release of self-antigens and a subsequent autoimmune response [313]. Moreover, the inflammatory factors secreted by activated immune cells may lead to immune-mediated damage in organs predisposed to autoimmune diseases, as the levels of peripheral cytokines are often elevated posttreatment in patients suffering from irAEs [314, 315]. On the other hand, off-target effects may explain some types of irAEs. For example, CTLA-4 is also expressed on hypothalamic and pituitary tissues; thus, the administration of anti-CTLA-4 therapy would probably elicit hypophysitis [316]. Therefore, although corticosteroids remain the first-line management approach for irAEs, the underlying mechanisms may still vary among organs and specific conditions; thus, precise immunohistopathological results are necessary to guide the prescription of appropriate immunomodulatory biological agents, especially when corticosteroids are ineffective [309].
5.2 Cancer immunotherapy strategies: enhancement versus normalization
Among the different types of cancer immunotherapy methods, anti-PD therapy has achieved the most extensive success. Consequently, researchers have begun to reconsider the strategies underlying the development of cancer immunotherapy.
Based on the understanding of the cancer-immunity cycle [317], various types of immunotherapies were developed before anti-PD therapy. As mentioned above, those therapies generally activate the immune system and enhance the antitumor response. However, these general approaches do not aim to correct any deficiencies in the normal antitumor immune response. They simply activate the total immune response and push the immune system to supraphysiological levels to constrain tumors [38]. Therefore, the objective tumor response rates of these strategies are usually low, while the risk of accompanying irAEs is rather high. These general approaches were later categorized together as ‘‘enhancement cancer immunotherapy’’ by the team of Professor Lieping Chen [38].
In contrast, based on the understanding that tumors can develop various ways to escape elimination by the immune system [318], whereby tumor cells inhibit immune cell activity in the TME, which results in a local but not systemic immunosuppression status [319], new approaches, such as anti-PD therapy, have been developed. These new approaches were categorized and termed “normalization cancer immunotherapy” because they aimed to restore a lost antitumor immune response [38]. These new strategies target a tumor-induced immune escape mechanism, selectively modulating and resetting immunity in the TME. A clear example is anti-PD therapy, which blocks the PD-1/PD-L1 pathway, a tumor-induced immune escape mechanism, to restore antitumor immunity in the TME without leading to general activation of the immune system. This approach has been proved to be effective across multiple cancer types and to be associated with a relatively low risk of accompanying irAEs. Therefore, Sanmamed et al. [38] proposed a strategic shift from enhancement to normalization in cancer immunotherapy development and emphasized the importance of identifying the critical immune escape mechanism in each patient.
Targeting SIGLEC-15, another TME-specific immune checkpoint target mentioned above, was inspired by this conceptual shift [85]. A monoclonal antibody that blocks SIGLEC-15-mediated immune suppression showed encouraging single-agent antitumor activity in a phase I clinical trial [320]. Therefore, we believe that in addition to PD-1/PD-L1, SIGLEC-15 may be the first in a series of novel targets for normalization cancer immunotherapy, which would progress cancer treatment to another level.
5.3 Hyperprogressive disease: the dark side of anti-PD therapy
Although anti-PD therapy can substantially improve the survival of patients with advanced cancers, there is a paradoxical phenomenon whereby some patients exhibit accelerated disease progression when treated with anti-PD-1/PD-L1 antibodies and have subsequent dramatically reduced survival durations, namely, hyperprogressive disease (HPD) [321].
HPD after anti-PD therapy was first reported at the 2016 European Society for Medical Oncology (ESMO) meetings by Lahmar et al. [322] who observed eight HPD cases (9%) in a cohort of 89 NSCLC patients receiving anti-PD therapy. Later, Champiat et al. [323] demonstrated that HPD could also occur in other examined cancer types, including melanoma, urothelial carcinoma, colorectal carcinoma, lymphoma, ovarian carcinoma, cholangiocarcinoma, and uveal melanoma. Additionally, in clinical trials comparing anti-PD therapy with chemotherapy, a crossover of the two survival curves was observed in the initial three to six months, which was considered to be due to disease progression and death occurring at a higher proportion in the anti-PD therapy arm within the early period of the trials [41, 324–327].
Researchers have expended great efforts to explore the biological mechanism of HPD, which is not just the manifestation of primary resistance to anti-PD therapy. It is accelerated tumor growth triggered by the administration of anti-PD-1/PD-L1 antibodies through undefined mechanisms. Several hypotheses regarding the HPD mechanism have been proposed, including: i) the expansion of PD-1-expressing Tregs due to the loss of contrasuppression, thus, leading to enhanced immunosuppression and tumor boosting; ii) the exhaustion of compensatory T cells due to the compensatory upregulation of the expression of other immune checkpoint molecules; iii) the modulation of tumor-promoting cells and secretion of immunosuppressive cytokines; iv) aberrant inflammation mediated by Th1 and Th17 cells; and v) the activation of an oncogenic signaling pathway [321]. However, evidence supporting these possible biological mechanisms is mostly preliminary and requires further investigation, which would certainly provide a better understanding of HPD and an approach for the management of this condition.
On the other hand, predictive biomarkers that identify people at high risk of HPD are also urgently needed. Patients with a local recurrence of head and neck squamous cell carcinoma (HNSCC) in the field of irradiation [328], NSCLC patients with two or more metastatic sites [329], and older patients are reported to be at a higher risk of HPD [323]. With regard to genomic biomarkers, MDM2 amplification was the first genomic biomarker reported to be associated with an increased risk of HPD [330]; in addition, EGFR alteration and 11q13 amplification were also reported to be associated with HPD [330, 331]. In summary, additional predictive biomarkers are needed, and the comprehensive integration of these biomarkers may lead to the identification of patients susceptible to HPD.
In addition to the passive clinical management of HPD such as informing patients, paradigm changes in tumor assessment and the choice of subsequent therapy [321], splicing in a limited course of chemotherapy to immunotherapy could potentially provide rapid disease control, making it a practical method to tackle HPD (Figure 4). In CheckMate 9LA, a phase 3 clinical trial evaluating nivolumab plus ipilimumab plus a limited course of chemotherapy versus chemotherapy for first-line treatment of stage IV or recurrent NSCLC, the crossover of the survival curves was eliminated by adding the limited course of chemotherapy compared with the results of CheckMate 227 [41]. Although Reck et al. [332] did not report HPD data for CheckMate 9LA at the 2020 American Society of Clinical Oncology (ASCO) meetings, the proportion of patients with disease progression during the first evaluation in the experimental arm was shown to be lower than that in the control arm (9% versus 13%). Therefore, the use of a limited course of chemotherapy to address HPD is worthy of further investigation.
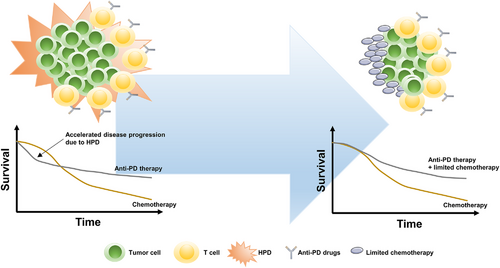
Adding a limited course of chemotherapy to immunotherapy to address HPD. Accelerated disease progression due to HPD significantly compromised the total survival benefit of anti-PD therapy over chemotherapy, which could be rescued by adding a limited course of chemotherapy to anti-PD therapy. Abbreviations: HPD: hyperprogressive disease; PD: programmed death
In summary, preferred cancer immunotherapy must have prominent response but less toxicity, such as anti-PD therapy. More efficient and novel targets will be developed in accordance with the strategy of normalization cancer immunotherapy. Nevertheless, special features such as fatal irAEs and HPD should be carefully managed for better application of cancer immunotherapy.
6 CONCLUSION
The current oncotherapy has being quickly evolved with the rise of antitumor immunotherapy. We are in an epoch with many opportunities for lengthening the lifespan of patients suffering from cancer. With the development of precision medicine, more appropriate individual treatments based on the comprehensive analysis of both tumors and the TME can be offered to patients . However, opportunities are always accompanied by challenges. In 2020, Hegde et al [333] listed the 10 largest challenges in cancer immunotherapy, covering issues ranging from preclinical experiments to therapeutic endpoints, that indicated the existence of insufficient recognition in cancer-related immunity. Despite all the setbacks in this field, we still hold full confidence in the potential of immunotherapy which can be realized with the use of more advanced medical devices and newly developed experimental methods. Immunity is considered either the weapon or the armor with which humans are born, so the mobilization and usage of this system should be an ideal option for disease control. When treating cancer with new therapeutic methods, we should not only pay attention to the dynamic alterations in the TME but also consider the relationship between local lesions and the basic status of patients. As scientists gradually expand the knowledge of immunotherapy, medical research and cancer treatments will likely advance significantly in the next decade.
DECLARATIONS
AUTHORS’ CONTRIBUTIONS
Conception: all authors. Manuscript writing: Y Wang, M Wang and HXW. Manuscript revision: RHX. All authors read and approved the final manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
ACKNOWLEDGMENTS
We thank Professor Feng Wang, Hai-Yan Zhang and Xia Zhao, Doctor Jia-Bo Zheng, Zi-Xian Wang and Jia-Huan Lu for their valuable scientific advice and discussion. This work was supported by grants from the National Natural Science Foundation of China (81930065).
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.