Pre-transplantation cytoreduction does not benefit advanced myelodysplastic syndrome patients after myeloablative transplantation with grafts from family donors
Abstract
Background
The role of pre-hematopoietic stem cell transplantation (HSCT) cytoreduction with either induction chemotherapy (IC) or hypomethylating agents (HMAs) in treating advanced myelodysplastic syndrome (MDS) remains debatable. We aimed to evaluate pre-HSCT strategies by comparing the endpoints related to disease control between advanced MDS patients with pre-HSCT cytoreduction and those with best supportive care.
Methods
We described 228 consecutive advanced MDS patients who received HSCT from a haploidentical donor (HID, n = 162) or matched related donor (MSD, n = 66) with uniform myeloablative conditioning regimens between January 2015 and December 2018. Of these 228 patients, 131 (57.5%) were treated exclusively with pre-HSCT best supportive care (BSC), 49 (22.5%) were given HMA, and 48 (21.1%) received both IC and HMA. Propensity score-matching analysis, multivariate analyses, and subgroup analyses were performed to elucidate the impact of pre-HSCT strategies on transplant outcomes.
Results
The 3-year relapse-free survival (RFS) rates were 78.2% and 70.0% for the BSC and cytoreduction cohorts (P = 0.189) and were 78.2%, 66.7%, and 73.2% for the BSC, HMA, and HMA+IC groups, respectively (P = 0.269). A propensity score-matching analysis confirmed that the 3-year RFS rates were 81.9%, 87.5%, and 66.9% for BSC, cytoreduction complete remission (CR), and cytoreduction non-CR groups, respectively (P = 0.051). Multivariate analyses demonstrated that pre-HSCT cytoreduction, older patient age, monosomal karyotype, and interval between diagnosis and HSCT were poor prognostic factors for RFS. In the subgroup analyses, BSC was associated with longer RFS compared to cytoreduction among the younger patients, those with international prognostic scoring system intermediate-2/high risk at diagnosis, and those with intermediate/poor cytogenetics.
Conclusions
Different pre-HSCT therapies did not yield discrepant post-HSCT outcomes. No benefit in terms of post-HSCT outcomes were correlated with pre-HSCT cytoreduction in advanced MDS even for cytoreduction CR patients. Early referral to HSCT is essential for advanced MDS patients.
Abbreviations
-
- allo-HSCT
-
- allogeneic hematopoietic stem cell transplantation
-
- BM
-
- bone marrow; BSC: best supportive care
-
- CK
-
- complex karoytype
-
- CR
-
- complete remission
-
- GVHD
-
- graft-versus-host-disease
-
- HID
-
- haploidentical donor
-
- HIDT
-
- haploidentical donor transplantation
-
- HLA
-
- human leukocyte antigen
-
- HMA
-
- hypomethylating agents
-
- IC
-
- induction chemotherapy
-
- IPSS
-
- International Prognostic Scoring System
-
- IWG
-
- International Working Group
-
- MAC
-
- myeloablative conditioning
-
- MCR
-
- marrow complete remission
-
- MDS
-
- myelodysplastic syndrome
-
- MK
-
- Monosomal karyotype
-
- MSD
-
- matched sibling donor
-
- MSDT
-
- matched sibling donor transplantation
-
- NR
-
- non-responders
-
- OR
-
- responders
-
- OS
-
- overall survival
-
- PD
-
- progressive disease
-
- PR
-
- partial remission
-
- RFS
-
- relapse-free survival
-
- RIC
-
- reduced-intensity conditioning
-
- SD
-
- stable disease
-
- URD
-
- matched unrelated donor
-
- URDT
-
- matched unrelated donor transplantation
-
- WHO
-
- World Health Organization
1 BACKGROUND
Hematopoietic stem cell transplantation (HSCT) is a curative therapy for myelodysplastic syndrome (MDS) [1-4]. Pre-HSCT cytoreduction either with induction chemotherapy (IC) or hypomethylating agents (HMA) for advanced MDS has been applied to lower the risk of post-HSCT relapse, but the advantage for long-term outcomes after HSCT is uncertain due to the absence of prospective, controlled trials. On the other hand, pre-HSCT cytoreduction, which is associated with prolonged myelosuppression, infection, and organ toxicities rendered a great proportion of patients ineligible for curative allogeneic HSCT (allo-HSCT) [5-7]. Voso et al. [5] reported that only 56% of patients treated with azacytidine had undergone HSCT. Previous studies demonstrated comparable outcomes between pre-HSCT IC and HMA [6, 8-10]. However, these studies were limited by comparing one type of cytoreductive approach versus another [8-10], rather than directly evaluating the impact of cytoreduction versus upfront HSCT with best supportive care (BSC) on post-HSCT outcomes.
There is insufficient information to help assess the selection of pre-HSCT cytoreduction and whether it could benefit advanced MDS patients undergoing allo-HSCT. The selection process for pre-HSCT cytoreduction is complicated and is based on the disease biology, the outcomes of various therapies, and the influence of responses to the “bridge” therapy on post-HSCT outcomes. Several previous reports revealed similar survival after HMA or IC versus BSC for MDS [11-13]. Although these limited data have shown no beneficial effect of pre-HSCT cytoreduction on post-HSCT outcomes, these results cannot determine whether BSC or cytoreduction should be applied for a specific patient due to the unbalanced factors between study groups, the highly diverse populations that included all MDS stages over a long period (more than 10 years), various HSCT regimens including reduced intensity conditioning and myeloablative conditioning, and different graft-versus-host disease (GVHD) prophylaxis [11-13].
Studies have been performed on patients with advanced MDS. Schroeder et al. [14] reported that upfront HSCT for higher-risk MDS patients, who were enrolled during a 17-year period, receiving either myeloablative conditioning or reduced intensity conditioning is not inferior to pre-HSCT cytoreduction in terms of survival. All these studies were confined to human leukocyte antigen (HLA)-matched sibling donor (MSD) or HLA-matched unrelated donor (URD) HSCT. Apart from heterogeneous disease stages and HSCT modalities, the differences in post-HSCT outcomes between cytoreduction and BSC might also be associated with different donor sources. Our recent study revealed that, in a subgroup of advanced MDS patients receiving myeloablative conditioning either from matched sibling donor transplantation (MSDT) or haploidentical donor transplantation (HIDT), relapse-free survival (RFS) rate was higher in patients with BSC than in those with prior cytoreduction (67% vs. 57%, P = 0.03) [3]. Although this study included a population with more homogenous disease stages, there were some drawbacks in this analysis. First, it did not discriminate between HMA and IC, and it did not evaluate the depth of response to cytoreduction according to the International Working Group (IWG) criteria due to the lack of detailed information from registry-based data. Second, the study enrolled patients over a 10-year period from multiple centers during which supportive care could have changed considerably and center effects (depend on the size and experience of each transplantation center in managing post-HSCT complications, life-threatening infections, and relapse) could not be excluded. Considering these limitations, matched-pair studies and more solid evidence of cytoreduction being not superior to upfront HSCT are needed to challenge international expert recommendations to administer pre-HSCT cytoreduction in higher-risk MDS patients [1, 15, 16].
Herein, in the present study, we aimed to evaluate pre-HSCT strategies by comparing the outcomes between advanced MDS patients who underwent either HIDT or MSDT with cytoreduction and those who received BSC only. Furthermore, propensity score matching (PSM) was conducted to reduce or eliminate the confounding factors between groups. In addition, subgroup analyses of different age groups, disease risks, cytogenetics, and donor sources were also performed.
2 PATIENTS AND METHODS
2.1 Patient selection
We enrolled consecutive MDS patients with excess blasts undergoing HSCT with grafts from family donors at our institution between January 2015 and December 2018. We included patients with ≥5% bone marrow (BM) blasts at peak, since this cut-off represents a potential trigger for pre-HSCT cytoreduction. Other inclusion criteria included sufficient cardiac function, lung diffusion capacity of at least 80%, transaminases level less than three times of the upper limit of normal, and creatinine clearance greater than 70 mL/min. Patients were excluded from HSCT if they had any active infections, transformation to acute myeloid leukemia, received URD HSCT, or received IC only before HSCT. Disease stages [refractory anemia with excess blasts (RAEB)-1 or RAEB-2] were classified based on the French-American-British (FAB) criteria [17]. Patients with ≥20% myeloblasts, defined as RAEB-t by the FAB criteria [17] but defined as acute leukemia by the world health organization [18], were excluded. Cytogenetic risk was defined based on the International Prognostic Scoring System (IPSS) criteria [19] and the revised IPSS (IPSS-R) criteria [20]. A monosomal karyotype (MK) was defined according to a published report [21]. All patients gave informed consent in accordance with the Declaration of Helsinki and according to the rules directed by the Institutional Review Board of Peking University People's Hospital.
The decision on the administration of pre-HSCT cytoreduction or BSC was made individually for each patient, mainly depending on the referral time. Patients referred to our center at diagnosis were intended to undergo upfront HSCT. In contrast, for patients referred to our center during the disease course, the referring physician made the decision on pre-HSCT treatment. In other words, most patients in the cytoreduction cohort received pre-HSCT cytoreduction at other hospitals prior to referral.
2.2 Prior therapy and response evaluation
Patients in the BSC cohort received only BSC (blood transfusions and growth factors). Patients in the cytoreduction cohort were given at least one cycle of HMA or HMA in combination with IC. IC regimens included at least the joint use of cytarabine and an anthracycline. Patients receiving IC only were excluded. According to the IWG criteria [22], complete remission (CR) and marrow complete remission (MCR) at the time of HSCT were grouped together as overall response (OR), while partial remission (PR), stable disease (SD), and progressive disease (PD) at the time of HSCT were grouped together as non-response (NR).
2.3 Donor selection and HLA typing
An MSD was the first choice for allo-HSCT. If an MSD was unavailable, subjects without a suitable closely HLA-matched URD (>8 of 10 matching HLA-A, B, C, DR, and DQ loci and > 5 of 6 matching HLA-A, B, and DR loci) or whose disease state left insufficient time for a URD searching were eligible for HIDT. HLA typing has been previously described in details [3].
2.4 HSCT procedure
For HIDT patients, the uniform conditioning regimen consisted of cytarabine (4 g/m2, intravenous infusion, days -10 to -9; Pfizer Pharmaceuticals Ltd., New York, NY, USA), busulfan (3.2 mg/kg, intravenous infusion, days -8 to -6; Otsuka Pharmaceutical Co. Ltd., Naruto, Tokushima, Japan), cyclophosphamide (1.8 g/m2, intravenous infusion, days -5 to -4; Baxter Oncology GmbH, Frankfurt, Hesse-Darmstadt, Germany), semustine (250 mg/m2, oral administration, day -3; ZheJiang Ruixin Pharmaceutical Co. Ltd., Lishui, Zhejiang, China), and rabbit antithymocyte globulin (2.5 mg/kg, intravenous infusion, days -5 to -2; Imtix Sangstat, Lyon, France). MSDT patients received hydroxycarbamide (80 mg/kg, oral administration, day -10; Qilu Pharmaceutical Co. Ltd., Jinan, Shandong, China) and a lower dose of cytarabine (2 g/m2, intravenous infusion, day -9) without antithymocyte globulin, and received the same other agents as in the HIDT regimen. GVHD prophylaxis was comprised of cyclosporine (2.5 mg/kg, intravenous infusion, since day -9), mycophenolate mofetil (1.0 g, oral administration, since day -9), and short-course methotrexate (15 mg/m2, intravenous infusion, on day 1 and 10 mg/m2 on days 3, 6, and 11) for all patients. Granulocyte colony stimulating factor (5 μg/kg) mobilized bone marrow and peripheral blood grafts were infused on the day of collection for HSCT. The total target mononuclear cell count for bone marrow and peripheral blood grafts was ≥6 × 108/kg recipient weight. Details of the HSCT procedure have been previously described [23].
2.5 Follow-up
The follow-up period ended by March 1, 2020. Bone marrow examinations were scheduled at 1, 2, 3, 4.5, 6, 9, and 12 months after HSCT and at 6-month intervals thereafter.
2.6 Study endpoints and definitions
The primary endpoint was relapse-free survival (RFS). The secondary endpoints included rates of successful engraftment, relapse, non-relapse deaths, acute and chronic GVHD (aGVHD and cGVHD), and overall survival (OS). Assessments of endpoints have been previously described in details with all data calculated from the day of graft infusion [3]. Relapse was identified as hematologic recurrence of MDS based on standard criteria [22, 24].
2.7 Statistical analysis
The probabilities of OS and RFS were calculated using the Kaplan-Meier estimator with the log-rank test. Cumulative rates of successful engraftment, GVHD, relapse, and non-relapse deaths were estimated, taking into account competing risks. Competing events were defined as follows: for GVHD, successful engraftment, relapse, and death from any cause; for non-relapse deaths, relapse. Multivariate analyses were performed to identify independent predictors of non-relapse deaths, relapse, RFS, and OS. Pre-HSCT cytoreduction was included in the Cox proportional hazards model with forced entry modeling. Backward elimination with a criterion of P < 0.10 for retention was used to select a final model. The following variables were analyzed in the multivariate analysis: patient age, sex, disease characteristics (IPSS score, disease stage at peak, MK, blast count at HSCT), and HSCT-related variables (interval between diagnosis and HSCT, donor source, donor-recipient sex match). MK and complex karyotype (CK) were also included into the models due to their potential to distinguish risk groups for relapse, RFS, and OS in a linear fashion. Because pre-HSCT treatment with cytoreduction or BSC was not allocated through randomization, a 1:1 ratio PSM analysis was conducted using nearest neighbor or exact matching of age, sex, WHO disease stage, cytogenetics, interval between diagnosis and HSCT, and donor source. The SPSS software package (version 22.0, SPSS Inc., Chicago, IL, USA) and R statistical software (Bell Labs, New York, NY, USA) were used for data analyses.
3 RESULTS
3.1 Patients and pre-HSCT treatment
A total of 228 patients were enrolled. Among them, 162 (71.1%) underwent HIDT, and 66 (28.9%) underwent MSDT. Fifty-nine of the 162 patients receiving HIDT were previously reported [2] and further followed in this study. The median age was 42 years (range, 2-66 years). Among the 228 patients, 131 (57.5%) received solely BSC; 49 (21.5%) were given HMA, and 48 (21.1%) received both HMA and IC (HMA+IC). Before therapy, 42 (18.4%), 132 (57.9%), and 54 (23.7%) patients had intermediate-1, intermediate-2, and high-risk disease, respectively, per the IPSS criteria. Seventeen (7.5%) achieved CR, 56 (24.6%) achieved MCR, 135 (59.2%) had SD, and 20 (8.8%) had PD at the time of HSCT as defined by IWG criteria. In the cytoreduction cohort, the CR rate after pre-HSCT cytoreduction was 17.5% (4.1% for the HMA group and 31.3% for the HMA+IC group, P < 0.001), and the OR (CR+MCR) rate was 49.5% (36.7% for the HMA group and 62.5% for the HMA+IC group, P = 0.015).
Details on the characteristics of the patients and donors in the BSC and the cytoreduction cohorts are presented in Table 1. Patients in the cytoreduction cohort had a higher rate of refractory anemia with excess blasts subtype 2 and higher BM blast counts both at peak and at HSCT than patients in the BSC cohort (all P < 0.05).
| Cytoreduction cohort | |||||
|---|---|---|---|---|---|
| Characteristic | HMA | IC + HMA | Total | BSC cohort | P value |
| Total (cases) | 49 | 48 | 97 | 131 | |
| Patient age [years, median (range)] | 44 (19-60) | 40 (5-63) | 42 (5-63) | 43 (6-64) | 0.599 |
| Patient gender [cases (%)] | 0.403 | ||||
| Male | 39 (79.6) | 24 (50.0) | 63 (64.9) | 77 (58.8) | |
| Female | 10 (20.4) | 24 (50.0) | 34 (35.1) | 54 (41.2) | |
| Disease stage* [cases (%)] | 0.020 | ||||
| RAEB1 | 19 (38.8) | 11 (22.9) | 30 (30.9) | 61 (46.6) | |
| RAEB2 | 30 (61.2) | 37 (77.1) | 67 (69.1) | 70 (53.4) | |
| Karyotype [cases (%)] | 0.022 | ||||
| Normal | 27 (55.1) | 27 (56.3) | 54 (55.7) | 51 (38.9) | |
| Monosomal | 3 (6.1) | 5 (10.4) | 8 (8.2) | 17 (13.0) | |
| Complex | 2 (4.1) | 1 (2.1) | 3 (3.1) | 15 (11.5) | |
| Other abnormal | 17 (34.7) | 15 (31.3) | 32 (33.0) | 48 (36.6) | |
| TP53 mutations [cases (%)] | 0.676 | ||||
| Present | 1 (2.0) | 1 (2.1) | 2 (2.1) | 3 (2.3) | |
| Absent | 13 (26.5) | 11 (22.9) | 24 (24.7) | 26 (19.8) | |
| Not detected | 35 (71.4) | 36 (75.0) | 71 (73.2) | 102 (77.9) | |
| IPSS risk group [cases (%)] | 0.239 | ||||
| Intermediate-1 | 10 (20.4) | 5 (10.4) | 15 (15.5) | 27 (20.6) | |
| Intermediate-2 | 26 (53.1) | 28 (58.3) | 54 (55.7) | 78 (59.5) | |
| High | 13 (26.5) | 15 (31.3) | 28 (28.9) | 26 (19.8) | |
| IPSS poor-risk cytogenetics #[cases (%)] | 2 (4.1) | 9 (18.8) | 11 (11.3) | 34 (26.0) | 0.007 |
| IPSS-R risk group [cases (%)] | 0.455 | ||||
| Intermediate | 7 (14.3) | 5 (10.4) | 12 (12.4) | 17(13.0) | |
| High | 17 (34.7) | 15 (31.3) | 32(33.0) | 53 (40.5) | |
| Very high | 25 (51.0) | 28 (58.3) | 53 (54.6) | 61 (46.6) | |
| IPSS-R poor/very poor cytogenetics $[cases (%)] | 2 (4.1) | 6 (12.5) | 8 (8.2) | 34 (26.0) | 0.001 |
| Interval between diagnosis and HSCT [months, median (range)] | 7 (2-96) | 6 (2-24) | 6 (2-96) | 6 (1-360) | 0.894 |
| Blast count [%, median (range)] | |||||
| At peak | 11 (5-19) | 13 (5-20) | 13 (5-20) | 10 (5-20) | 0.016 |
| At HSCT | 5 (0-19) | 3 (0-17) | 5 (0-19) | 8 (0-20) | <0.001 |
| Blast count at peak [cases (%)] | 0.019 | ||||
| <10% | 19 (38.8) | 10 (20.8) | 29 (29.9) | 60 (45.8) | |
| ≥10% | 30 (61.2) | 38 (79.2) | 68 (70.1) | 71 (54.2) | |
| Blast count at HSCT [cases (%)] | <0.001 | ||||
| <5% | 18 (36.7) | 31 (64.6) | 49 (50.5) | 28 (21.4) | |
| ≥5% | 31 (63.3) | 17 (35.4) | 48 (49.5) | 103 (78.6) | |
| Donor sex [cases (%)] | 1.000 | ||||
| Male | 28 (57.1) | 34 (70.8) | 62 (63.9) | 83 (63.4) | |
| Female | 21 (42.9) | 14 (29.2) | 35 (36.1) | 48 (36.6) | |
| Donor source [cases (%)] | 0.883 | ||||
| Haploidentical donor | 32 (65.3) | 36 (75.0) | 68 (70.1) | 94 (71.8) | |
| Matched sibling donor | 17 (34.7) | 12 (25.0) | 29 (29.9) | 37 (28.2) | |
| Follow-up [months, median (range)] | 28 (0.4-55) | 21 (1-61) | 22 (0.4-61) | 26 (0.1-61) | 0.225 |
| Survival status [(cases) (%)] | |||||
| Survival | 36 (73.5) | 37 (77.1) | 73 (75.3) | 101 (77.1) | |
| Death | 13 (26.5) | 11 (22.9) | 24 (24.7) | 26 (19.8) | |
| Lost to follow-up | 0 | 0 | 0 | 4 (3.1) | |
- * Disease stage was classified according to the French-American-British criteria [17].
- # IPSS poor risk is defined as complex karyotype with at least 3 abnormalities or chromosome 7 anomalies.
- $ IPSS-R poor risk is defined as -7, inv(3)/t(3q)/del(3q), double including-7/del(7q), complex karyotype with 3 abnormalities; IPSS-R very poor is defined as complex karyotype with more than 3 abnormalities.
- Abbreviations: HSCT, hematopoietic stem cell transplantation; HMA, hypomethylating agents; IC, induction chemotherapy; MDS, myelodysplastic syndrome; BSC, best supportive care; RAEB-1/-2, refractory anemia with excess blasts subtype 1/2; IPSS, International Prognostic Scoring System; IPSS-R, IPSS-revised;
3.2 Post-HSCT outcomes according to pre-HSCT therapy
As of the last follow-up, 50 patients had died, and 4 were lost to follow-up. The median follow-up period for all patients was 25.1 months (range, 0.1-61.1 months). The estimated rates of 3-year OS, 3-year RFS, relapse, and non-relapse deaths were 77.5% [95% confidence interval (CI): 71.9%-83.1%], 74.8% (95% CI: 69.0%-80.6%), 7% (95% CI: 3%-10%), and 17% (95% CI: 12%-22%), respectively.
The 3-year relapse rate was significantly lower in the BSC cohort than in the cytoreduction cohort [2.7% (95% CI: 0-5.7%) vs. 14.7% (95% CI: 6.7%-22.7%), P = 0.001], but no significant differences were observed in the rates of non-relapse deaths, 3-year RFS, and 3-year OS (all P > 0.05; Figure 1A-D). When stratifying the cytoreduction cohort into the HMA and HMA+IC groups, similar differences between the BSC cohort and the two groups were observed (overall P = 0.269 for RFS; P = 0.108 for BSC vs. HMA, and P = 0.610 for BSC vs. HMA+IC; Figure 1E.
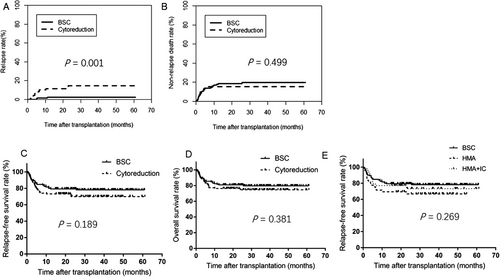
3.3 Outcome predictors
For patients with CK, the 3-year RFS rate was comparable between the BSC and cytoreduction cohorts [69.0% (95% CI: 53.8%-84.2%) vs. 62.5% (95% CI: 28.3%-96.7%), P = 0.381]. Among the 37 patients with CK, only 9 were tested for TP53 mutations: 3 with TP53 mutation (2 achieved RFS until the last follow-up) and 6 without (2 achieved RFS until the last follow-up). Regarding the donor type, the 3-year RFS rate was not significantly different between the MSDT and HIDT groups [83.3% (95% CI: 74.1%-92.5%) vs. 71.1% (95% CI: 63.7%-78.5%), P = 0.080]. Multivariate analysis confirmed that BSC was better than cytoreduction regarding OS and RFS with lower relapse and comparable non-relapse deaths (Table 2). Older age, MK, and longer interval between diagnosis and HSCT reduced both RFS and OS. Older age and longer interval between diagnosis and HSCT were related to increased non-relapse death rates.
| Variate | Relapse | Non-relapse deaths | Overall survival | Relapse-free survival | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Pre-HSCT treatment | ||||||||
| Cytoreduction | 1.000 | 1.000 | 1.000 | 1.000 | ||||
| BSC | 0.135 (0.037-0.498) | 0.003 | 0.761 (0.373-1.552) | 0.453 | 0.502 (0.268-0.942) | 0.032 | 0.493 (0.259-0.940) | 0.032 |
| Patient age | 1.005 (0.966-1.046) | 0.788 | 1.032 (1.004-1.061) | 0.024 | 1.031 (1.006-1.056) | 0.014 | 1.025 (1.003-1.048) | 0.026 |
| Patient sex | ||||||||
| Female | 1.000 | 1.000 | 1.000 | 1.000 | ||||
| Male | 0.776 (0.218-2.760) | 0.695 | 1.352 (0.671-2.722) | 0.398 | 1.211 (0.652-2.249) | 0.545 | 1.095 (0.606-1.981) | 0.763 |
| IPSS-R score | 0.802 | 0.338 | 0.379 | 0.728 | ||||
| Intermediate | 1.000 | - | 1.000 | - | 1.000 | - | 1.000 | - |
| High | 0.651 (0.110-3.841) | 0.635 | 2.556 (0.733-8.917) | 0.141 | 2.414 (0.698-8.347) | 0.164 | 1.495 (0.549-4.074) | 0.432 |
| Very high | 0.962 (0.196-4.723) | 0.962 | 2.201 (0.628-7.711) | 0.217 | 2.143 (0.609-7.550) | 0.235 | 1.342 (0.483-3.729) | 0.572 |
| Disease stage | ||||||||
| RAEB2 | 1.000 | 1.000 | 1.000 | 1.000 | ||||
| RAEB1 | 0.574 (0.153-2.157) | 0.411 | 1.778 (0.915-3.455) | 0.090 | 1.211 (0.652-2.249) | 0.544 | 1.265 (0.703-2.274) | 0.433 |
| Cytogenetics at diagnosis | 0.120 | 0.415 | 0.121 | 0.162 | ||||
| Normal karyotype | 1.000 | - | 1.000 | - | 1.000 | - | 1.000 | - |
| Monosomal | 4.442 (0.841-23.466) | 0.079 | 1.549 (0.704-3.408) | 0.277 | 3.199 (1.226-8.351) | 0.018 | 2.762 (1.335-5.711) | 0.045 |
| Complex karyotype | 2.409 (1.486-11.933) | 0.282 | 2.794 (0.812-9.260) | 0.103 | 1.445 (0556-3.751) | 0.450 | 1.716 (0.735-4.003) | 0.212 |
| Other abnormal | 0.530 (0.143-1.963) | 0.342 | 1.313 (0.435-3.969) | 0.629 | 1.176 (0.612-2.621) | 0.626 | 1.053 (0.562-1.973) | 0.872 |
| Blast count at HSCT | ||||||||
| ≥5% | 1.000 | 1.000 | 1.000 | 1.000 | ||||
| <5% | 0.436 (0.141-1.351) | 0.150 | 0.996 (0.503-1.975) | 0.992 | 0.816 (0.437-1.526) | 0.524 | 0.657 (0.362-1.193) | 0.168 |
| Interval between diagnosis and HSCT | 0.985 (0.944-1.028) | 0.484 | 1.010 (1.004-1.015) | 0.001 | 1.009 (1.003-1.015) | 0.002 | 1.009 (1.003-1.015) | 0.003 |
| Donor source | ||||||||
| HID | 1.000 | 1.000 | 1.000 | 1.000 | ||||
| MSD | 0.949 (0.309-2.911) | 0.926 | 0.492 (0.187-1.295) | 0.151 | 0.722 (0.341-1.531) | 0.396 | 0.601 (0.287-1.257) | 0.176 |
| Donor-recipient sex | ||||||||
| Mismatch | 1.000 | 1.000 | 1.000 | 1.000 | ||||
| Match | 1.615 (0.533-4.893) | 0.397 | 1.448 (0.732-2.862) | 0.282 | 1.059 (0.585-1.917) | 0.851 | 0.958 (0.546-1.682) | 0.882 |
- Abbreviations: MDS, myelodysplastic syndrome; BSC, best supportive care; RAEB, refractory anemia with excess blasts; IPSS-R, International Prognostic Scoring System-revised; HSCT, hematopoietic stem cell transplantation; MSD, matched sibling donor; HID, haploidentical donor; HR, hazard ratio; CI, confidence interval.
3.4 PSM analysis
We pair-matched 91 BSC with 91 pre-HSCT cytoreduction cases. The 3-year RFS rate was comparable between the two cohorts (81.9% vs. 70.1%, P = 0.070, Figure 2A) and among three treatment groups (BSC 81.9%, HMA 67.4%, and HMA+IC 72.7%, P = 0.137, Figure 2B).
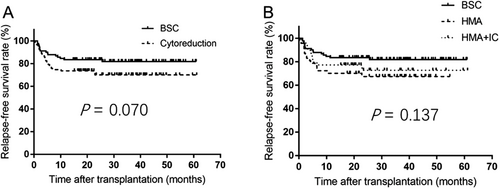
3.5 Post-HSCT outcomes according to response to pre-HSCT treatment or blast count in the PSM dataset
Of the 91 patients who received pre-HSCT cytoreduction, 16 achieved CR at HSCT (CR group), and 75 did not (non-CR group). We compared the post-HSCT outcomes of these two groups with those of the BSC cohort in the PSM dataset. The 3-year RFS rates were 81.9%, 87.5%, and 66.9% in the BSC, CR, and non-CR groups, respectively (overall P = 0.051). The RFS was shorter in the non-CR group than in the BSC cohort (HR 2.003; 95% CI, 1.064-3.772; P = 0.031), while no difference was noted between the CR group and the BSC cohort (HR 0.725; 95% CI, 0.166-3.155; P = 0.668). Furthermore, in the cytoreduction cohort, 44 patients achieved OR (CR + MCR) at HSCT (OR group), and 47 had no response (NR group). The 3-year RFS rates were 81.9%, 77.3%, and 65.2% in the BSC, OR, and NR groups, respectively (overall P = 0.108; P = 0.411 for BSC vs. OR, and P = 0.033 for BSC vs. NR).
Since a panel of international experts has recently advised to conduct pre-HSCT cytoreduction at least in those MDS patients with a BM blast count of ≥10% [1], we compared the outcomes between patients with a BM blast count of <10% (n = 30) and ≥10% (n = 61) at peak in the BSC cohort. We found no difference in 3-year RFS rate (81.4% vs. 82.0%, P = 0.840). By dichotomizing the patients according to the cut-off of 5% blasts at HSCT, we observed no difference in 3-year RFS rate in the pooled population (80.0% vs. 74.3%, P = 0.575), while the difference was significant in the cytoreduction cohort (82.2% vs. 59.8%, P = 0.046).
3.6 Subgroup analysis in the PSM dataset
To further examine the influence of pre-HSCT cytoreduction on post-HSCT outcomes, we performed subgroup analyses with stratification by age, donor source, IPSS risk, and cytogenetic risk. The 3-year RFS rate was significantly higher in the BSC cohort than in the cytoreduction cohort among patients younger than 45 years, in patients with IPSS intermediate-2/high risk, and in patients with IPSS intermediate/poor-risk cytogenetics (all P < 0.05, Table 3).
| No. of patients (cases) | 3-year RFS rate [%, estimate (95% CI)] | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Pooled population | BSC cohort | Cytoreduction cohort | Pooled population | BSC cohort | Cytoreduction cohort | P value |
| Age (years) | |||||||
| <45 | 95 | 46 | 49 | 79.3 (70.7-87.9) | 91.3 (82.9-99.7) | 65.3 (51.7-78.9) | 0.002 |
| ≥45 | 87 | 45 | 42 | 74.1 (64.1-84.1) | 72.1 (58.1-86.1) | 75.8 (62.2-91.4) | 0.548 |
| Donor source | |||||||
| MSD | 55 | 28 | 27 | 83.6 (73.6-93.6) | 92.7 (82.7-100) | 74.1 (57.3-90.9) | 0.059 |
| HID | 127 | 63 | 64 | 76.4 (68.8-84.0) | 76.9 (65.9-87.9) | 68.0 (55.4-80.6) | 0.318 |
| IPSS risk | |||||||
| Intermediate-1 | 31 | 16 | 15 | 83.6 (70.2-97.0) | 80.8 (60.8-100) | 86.7 (69.1-100) | 0.639 |
| Intermediate-2/high | 151 | 75 | 76 | 74.5 (67.1-81.9) | 82.1 (73.1-91.1) | 66.7 (55.3-78.1) | 0.037 |
| IPSS cytogenetics | |||||||
| Good | 105 | 54 | 51 | 79.0 (70.8-87.2) | 80.6 (69.4-91.8) | 77.2 (64.8-89.6) | 0.655 |
| Intermediate/poor | 77 | 37 | 40 | 72.1 (61.7-82.5) | 83.8 (71.6-96.0) | 61.2 (45.2-77.2) | 0.040 |
- Abbreviations: BSC, best supportive care; IPSS, International Prognostic Scoring System; MSD, matched sibling donor; HID, haploidentical donor
4 DISCUSSION
Our results of 228 consecutive advanced MDS patients revealed that the OS, RFS, relapse, and non-relapse deaths of patients who received only pre-HSCT BSC were at least not inferior to those of patients who had been given pre-HSCT cytoreduction even when compared with those of patients achieving CR after cytoreduction. These results were confirmed with the PSM analysis.
The role of pre-HSCT cytoreduction either with AML-like IC or HMA in patients with advanced MDS is still uncertain because of the paucity of randomized trials. Previously published patient populations were highly heterogeneous regarding disease stage, IPSS or IPSS-R risk stage, or conditioning intensity and were recruited during a period of more than 10 years [11-14, 25,26], thereby hindering a direct comparison. Our results, which were proven in multivariate analysis, the PSM dataset analysis, and subgroup analyses, support that pre-HSCT cytoreduction has comparable outcomes with BSC [27]. This was also validated when focusing on those patients with ≥10% BM blasts at peak. Furthermore, RFS in the BSC cohort did not vary between patients with BM blast of <10% and ≥10%. All these findings challenge a recent recommendation of cytoreduction for patients with > 10% BM blasts [1]. In addition, regardless of the pre-HSCT cytoreduction strategy (i.e., IC and/or HMA) or regimen, the RFS rate in the present study was relatively higher than the reported 3-year RFS rates approximately 40% (range, 36.6%-41.0%) [11-14, 25]. The reasons for the difference may be the younger patient age, the lower proportion of patients with CK/MK, and the homogenous myeloablative conditioning regimen in the current cohort compared with those in previous reports [11-14, 25].
Apart from the risk of being unable to proceed to HSCT due to rapid disease progression or a decline in performance status during pre-HSCT cytoreduction [5-7], another disadvantage is the insufficient CR rate after IC and/or HMA in patients with advanced MDS. In accordance with the CR rates reported to be approximately 30%-40% after IC [9, 10] and 7%-28% after HMA [5-8], we demonstrated a CR rate of 18% despite the median marrow blast at HSCT being 4% after cytoreduction. This implies that in case cytoreduction is considered, weighting the chance to of achieving CR according to the strict IWG criteria and blood cell count recovery against the risk of infections is vital.
The present study showed that patients with BSC attained comparable RFS to patients achieving CR at HSCT after cytoreduction. Interestingly, the patients with BM blasts below 5% after cytoreduction had better RFS than those with blasts above 5%, which indicates that treatment resistance and disease biology, rather than prior therapy, influence the HSCT outcomes. Previous publications have also documented consistent results [8, 11, 13]. In contrast, there were no meaningful variations in RFS between the patients with blasts counts of <5% and ≥5% among the whole population. This implies that the absolute disease burden at HSCT may not be the most influencing predictor of post-HSCT outcomes. Rather, the influence of the marrow blast before HSCT should be interpreted in lieu of the pre-HSCT treatment. Collectively, the response to cytoreduction, namely the chemo-sensitivity instead of disease burden at HSCT or the pre-HSCT treatment itself, has the most relevant effect on HSCT outcomes.
In addition to the influence of pre-HSCT treatment and the depth of response, other contributing factors are older age, poor cytogenetics, and a prolonged duration between diagnosis and HSCT. Older MDS patients have an increased rate of adverse-risk cytogenetics, poorer performance status, and more co-morbidities, rendering them to higher risk of relapse and non-relapse deaths and inferior survival [3, 21]. The negative impact of delayed HSCT for MDS patients has previously been confirmed [3, 8, 13]. In the subgroup analysis, we inferred that patients with older age or poor-risk disease characteristics may not benefit from pre-HSCT cytoreduction and that the potential adverse effect of cytoreduction may ultimately either hinder or delay them from proceeding to HSCT. Overall, advanced MDS patients should be transplanted early in the disease course.
Our analysis had limitations. Due to the retrospective nature of the study, unbalanced features existed between groups, although we made adjustments in the multivariate and PSM analyses. Another shortcoming was that somatic mutations and ferritin were not included in the study due to scanty data. Furthermore, the results of patients who underwent pre-HSCT cytoreduction but failed to proceeding to HSCT before being referred to our hospital could not be estimated in this retrospective study. Therefore, to circumvent the limitations of our study and all other published results, a randomized study is needed to determine the impact of the pre-HSCT cytoreduction strategy.
5 CONCLUSIONS
In conclusion, the currently established pre-HSCT therapies did not yield different post-HSCT outcomes among patients with advanced MDS irrespective of donor source, patient age, cytogenetics or IPSS risk. No benefit in outcomes was correlated with pre-HSCT cytoreduction in patients with advanced MDS, even in patients who achieved CR after pre-HSCT cytoreduction. Treatment resistance, rather than cytoreduction itself, is a poor prognostic marker. Early referral to HSCT is critical for advanced MDS patients.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study protocol was approved by the ethics committee of the local institution. Informed consent was obtained from all patients’ guardians. All authors vouch for the accuracy and completeness of the reported data and analyses.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The datasets analyzed during the current study are available from the corresponding authors on reasonable request.
COMPETING INTERESTS
The authors declare that they have no competing interests.
FUNDING
This work was partly supported by grants from the National Key Research and Development Program of China (2019YFC0840606) from the Ministry of Science and Technology, National Natural Science Foundation of China (Grant No. 82070189 & 81770189 & 81621001 & 81530046), Peking University Clinical Scientist Program (BMU2019LCKXJ003), the Fundamental Research Funds for the Central Universities, the Science and Technology Project of Guangdong Province of China (Grant No. 2016B030230003), and the project of health collaborative innovation of Guangzhou city (no. 201704020214), Beijing Municipal Science & Technology Commission (No. Z191100006619054).
AUTHORS' CONTRIBUTIONS
Y.W. and X-J.H. designed the research; Y.W. and Y-Q.S. analyzed the data and wrote the manuscript; and all authors, provided patient data, wrote the manuscript, and gave final approval for the manuscript.
ACKNOWLEDGEMENTS
We gratefully thank the patients and their families for participating in this study.




