Tumor heterogeneity and the potential role of liquid biopsy in bladder cancer
Abstract
Bladder cancer (BC) is a heterogeneous disease that characterized by genomic instability and a high mutation rate. Heterogeneity in tumor may partially explain the diversity of responses to targeted therapies and the various clinical outcomes. A combination of cytology and cystoscopy is the standard methodology for BC diagnosis, prognosis, and disease surveillance. However, genomics analyses of single tumor-biopsy specimens may underestimate the mutational burden of heterogeneous tumors. Liquid biopsy, as a promising technology, enables analysis of tumor components in the bodily fluids, such as blood and urine, at multiple time points and provides a minimally invasive approach that can track the evolutionary dynamics and monitor tumor heterogeneity. In this review, we describe the multiple faces of BC heterogeneity at the genomic and transcriptional levels and how they affect clinical care and outcomes. We also summarize the outcomes of liquid biopsy in BC, which plays a potential role in revealing tumor heterogeneity. Finally, we discuss the challenges that must be addressed before liquid biopsy can be widely used in clinical treatment.
Abbreviations
-
- APC
-
- APC regulator of WNT signaling pathway
-
- BC
-
- bladder cancer
-
- BRCA1
-
- BRCA1 DNA repair associated
-
- cfDNA
-
- cell-free DNA
-
- CT
-
- computed tomography
-
- CTCs
-
- circulating tumor cells
-
- ctDNA
-
- circulating tumor DNA
-
- EBCCs
-
- exudative bladder cancer cells
-
- EGFR
-
- epidermal growth factor receptor
-
- ERBB2
-
- receptor tyrosine-protein kinase erbB-2
-
- FGFR3
-
- fibroblast growth factor receptor 3
-
- FRɑ
-
- folate receptor ɑ
-
- GLOBOCAN
-
- Global Cancer Observatory: CANCER TODAY
-
- GSTP1
-
- glutathione s-transferase pi 1
-
- ITH
-
- Intra-tumoral heterogeneity
-
- lncRNA
-
- long non-coding RNA
-
- MIBC
-
- muscle-invasive bladder cancer
-
- miRNA
-
- microRNA
-
- NAC
-
- neoadjuvant chemotherapy
-
- NGS
-
- next-generation sequencing
-
- NMIBC
-
- non-muscle-invasive bladder cancer
-
- PD-L1
-
- programmed death-ligand 1
-
- PIK3CA
-
- phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha
-
- RAF1
-
- Raf-1 proto-oncogene
-
- TCGA
-
- The Cancer Genome Atlas
-
- TGFβ
-
- transforming growth factor β
-
- TIG1
-
- tazarotene-induced gene 1
-
- TP53
-
- tumor protein p53
-
- UC
-
- urothelial cancer
-
- UcfDNA
-
- urinary cell-free DNA
1 INTRODUCTION
Bladder cancer (BC) is the 10th most common cancer worldwide [1]. According to the Global Cancer Observatory: CANCER TODAY (GLOBOCAN), three quarters of all BC cases are predominantly male and more than 90% of patients are over 50 years old [2]. BC is a highly heterogeneous malignancy, especially in advanced stage [3]. Approximately 75% of BC patients are diagnosed with non-muscle-invasive bladder cancer (NMIBC), whereas, 10%-25% eventually develop muscle-invasive bladder cancer (MIBC) [4]. Patients with MIBC are offered cisplatin-based neoadjuvant chemotherapy (NAC), which may prolong median overall survival by 13-14 months with a response rate of 50% [5-7]. Non-response patients may lose the opportunity for additional therapy with their disease progression. The use of immune checkpoint blockade continues to break new ground in the management of MIBC, however, there are still many patients cannot benefit from these therapies [7, 8]. With the advent of precision oncology, more and more molecular subtyping is increasingly recognized in BC [9-13].
Several lines of evidence suggest that tumor heterogeneity occurs on multiple levels can lead to the distinct clinical outcomes of NMIBC and MIBC. For example, gain-of-function mutations of fibroblast growth factor receptor 3 (FGFR3) are more prevalent in low-grade NMIBC [14], whereas mutations in DNA damage repair genes [12, 15] and somatic mutations in receptor tyrosine-protein kinase erbB-2 (ERBB2) [16] were associated with an excellent response to NAC in patients with MIBC. Previous studies have proven that pancreatic ductal adenocarcinoma is the most common type of pancreatic cancer featured with high intra-tumoral heterogeneity (ITH) and poor prognosis [17], whereas other studies have shown a more modest level of ITH in lung cancer [18], or varying degree of ITH between patients with high-grade ovarian cancer [19]. However, studies in BC have shown a low level of ITH within individual biopsies, but a large difference between primary tumors and metastatic regions [20, 21]. The heterogeneity of BC results in great variation between different patients or regions of the same tumor tissue can lead to great differences in treatment efficacy and drug resistance [20, 21].
Since obtaining tumor tissue for biopsies is invasive and technically difficult, biopsies are limited to very few sampling points in time and accessible sites or metastatic sites, such as the lung, bone, and brain [22]. These limitations may fail to detect clinically relevant resistance mutations and pose a significant challenge to the development of BC treatment strategy [23]. The focus of precision oncology is increasingly turning to liquid biopsy. Liquid biopsy is used to analyze biomarkers in various body fluids, including blood, urine, and saliva [24-26]. It is noninvasive and can be repeated at multiple points in time. Recent studies have shown that attempts are now being made to use liquid biopsy as an alternative strategy to understand heterogeneity in different kinds of cancer, such as lung [27], breast [28], gastrointestinal [29, 30], and colorectal cancers [31]. With the application of liquid biopsy in BC gradually maturing in recent years, numerous studies have shown that liquid biopsy may play an important role in the management of patients with BC at different stages [32-35].
Here, we provide a comprehensive overview of BC heterogeneity at the genomic and transcriptional levels, which may predict disease progression and therapeutic response and could eventually affect clinical decision. We then discuss the current applications of liquid biopsy in BC research and analyze its potential for identifying tumor heterogeneity in clinical routine. The many challenges that need to be overcome in the future are also described.
2 HETEROGENEITY IN BC
Improved technology has allowed scientists and clinicians to characterize the heterogeneity of tumor at multiple levels. Moreover, multi-region sequencing was performed in many cancer types, including lung [36], breast [37], kidney [38-40], rectal [41], colorectal [42], prostate cancers [43], and BC [44], which provides the opportunity to expose the etiologies of treatment failure and drug resistance. At the molecular level, MIBC is a heterogeneous disease that is characterized by genomic instability and a high somatic mutation rate (median, 5.5 per megabase), similar to non-small cell lung cancer and melanoma [45]. This high mutation rate provides the fuel for tumor evolution and tumor heterogeneity, and eventually poses fundamental challenges for treatment remission. Therefore, it is essential to develop a comprehensive understanding of BC heterogeneity between tumors over time (Figure 1).
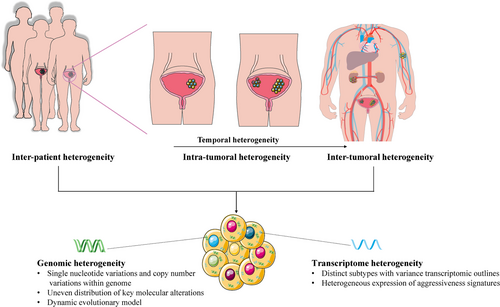
2.1 Genomic heterogeneity
Insights into the genomic landscape of MIBC, the consequences of heterogeneity at the individual level have now been well recognized by the different molecular subtypes. An analysis of 412 tumors and matched normal samples revealed that patients with high APOBEC-signature mutagenesis, which was strongly correlated with high mutation burden, showed a better overall survival than those with other mutational signatures [46]. Moreover, next-generation sequencing (NGS) of 178 cancer-associated genes in 110 MIBC patients has demonstrated that ERBB2 missense mutations are exclusively present in patients responding to NAC [16].
Current strategies for the treatment of MIBC or other cancers are typically relied on the biopsy of a single primary or metastatic site. In some multifocal cancers, such as breast [37], kidney [38], rectal [41], prostate cancers [43], and BC [44], the majority of point mutations detected in different fragments are frequently unique to a single fragment. These findings indicate that gene mutations found in single biopsies will not necessarily be representative of mutations presented in the entire tumor and ITH has substantial obstacle to appropriate selection of precision therapies. For example, in a recent study, Heide et al. [44] used multiregional whole-exome sequencing of 10 whole cystectomy specimens from BC to show an uneven distribution of key molecular alterations across distinct areas within a tumor. It intends to support an evolutionary model whereby synchronous development and parallel evolution of important alterations are followed in a dynamic rather than static way. This ongoing evolution might ultimately lead to inter-tumoral heterogeneity, meaning the differences between the primary tumor and metastases.
The transfer of disease from local organ to another part of the body is termed “metastasis”. Although distant metastasis is the leading cause of cancer-related deaths, current risk allocation and treatment recommendations for BC metastasis are primarily based on the histological and molecular characteristics of the primary tumor. Genomic studies in several types of tumors have revealed regionally diverse mutational landscapes between primary tumors and metastatic sites [47, 38, 48], and genetic differences between metastatic and primary tumors may affect treatment efficacy [49]. The study on the genetic difference between primary BC and local metastasis also showed that there were a distinct differences between primary tumor and metastasis in the degree of genetic differentiation [50, 20].
As multiregional sampling and blood-based liquid biopsies become widely studied, a high degree of genomic diversity across different regions (spatial heterogeneity) and over time (temporal heterogeneity) will be explained. However, the difference between primary tumor, metastatic, and circulating tumor markers currently remain to be elucidated.
2.2 Transcriptome heterogeneity
The classification of MIBC subtypes based on gene expression has become increasingly clear, and several reports have highlighted the clinical significance of molecular stratification of MIBC [51-55, 46, 56]. Seiler et al. [55] reported that patients with basal tumors should be prioritized for NAC, they found the first single-sample classifier to subtype MIBC, which may be suitable for integration into routine clinical practice. Moreover, patients with metastatic urothelial cancer (UC) and patients who responded to the anti-programmed death-ligand 1 (PD-L1) agent were associated with CD8+ T-effector cell phenotype and high neoantigen or tumor mutation burden. Lack of response was associated with a signature of transforming growth factor β (TGFβ) signaling in fibroblasts [57]. In recent years, The Cancer Genome Atlas (TCGA) database and other groups have identified multiple intrinsic subtypes of BC based on gene expression profiling (Table 1). These studies divided BC into distinct subtypes with variant transcriptomic outlines and the response to specific treatment types.
| Reference | Samples | Molecular subtype | Main findings |
|---|---|---|---|
| Robertson et al. [46] | 412 MIBC |
Luminal-papillary, Luminal-infiltrated, Luminal, Basal-squamous, Neuronal |
|
| Tan et al. [51] | 2411 NMIBC and MIBC |
Papillary-like, HER2-like, Luminal-like, Nerual-like, Mesenchymal-like, Squamous-cell carcinoma-like |
|
| Warrick et al. [52] | 309 BC co-occurring with conventional urothelial carcinomas |
Urothelia-like, Genomically unstable, Basal-squamous, Mesenchynal-like |
|
| Sjödahl et al. [54] | 307 MIBC |
Urothelial-like, Genomically unstable, Epithelial-Infiltrated, SCCL/Mesenchymal Infiltrated, SCCL/UroB, Small-cell/Neuroendocrine-like |
|
| Seiler et al. [55] | 305 MIBC |
Claudin-low, Basal, Luminal-infiltrated, Luminal |
|
| Efstathiou et al. [56] | 259 MIBC |
Luminal, Luminal-infiltrated, Basal, Claudin-low |
|
- Abbreviations: NMIBC, non–muscle-invasive bladder cancer; MIBC, muscle-invasive bladder cancer; PD-L1, programmed death-ligand 1; PD-1, programmed death-1; TP53, tumor protein p53; RB1, RB transcriptional corepressor 1; HER2, human epidermal growth factor receptor 2; SCCL, squamous-cell carcinoma-like; UroB, urothelial-like B; CR, complete response; PFS, progression-free survival; NAC, neoadjuvant chemotherapy; RC, radical cystectomy.
The study by Thomsen et al. [58] revealed inter-tumoral heterogeneity in various subtypes and aggressiveness signatures, and they also suggested that multiple subclones can occur within a MIBC patient. However, these subtype sets are not uniform and confusing. Kamoun et al. [59] performed a network-based analysis of six independent MIBC classification systems to reach a consensus on MIBC molecular subtypes (luminal papillary, luminal non-specified, luminal unstable, stroma-rich, basal/squamous, and neuroendocrine-like). Their results showed that the overall survival was directly associated with the subtypes. For example, patients with luminal papillary subtype tumors had a better outcome, and the neuroendocrine-like subtype tumors were associated with the worst prognosis. For guided therapy, basal/squamous subtype tumors expressed high levels of immune checkpoint markers and epidermal growth factor receptor (EGFR), which may be associated with sensitivity to immunotherapies and EGFR-targeted therapies [59]. Therefore, these studies demonstrated that responses to chemotherapy or immunotherapy may be enriched in specific MIBC subtypes.
3 NONINVASIVE MONITORING OF BC HETEROGENEITY
Practical molecular stratification of tumors will be critical to guide the use of emerging targeted therapies and immunotherapy in BC. However, in the setting of significant inter-tumoral heterogeneity or ITH, a single-site biopsy may not be representative for the entire tumor [41]. Moreover, longitudinal or simultaneous multi-site testing is not feasible because continuous tissue sampling is invasive and impinges on quality of life. Cystoscopy and urine cytology remain the gold standard for BC diagnosis, prognosis, and disease surveillance; unfortunately, cystoscopy is invasive and urine cytology is limited by its low sensitivity (20%-53%), especially in low-grade tumor [60]. Existing urinary biomarkers for BC, such as the nuclear matrix protein 22 [61, 62] and bladder cancer antigen, which are Food and Drug Administration-approved, are not widespread adopted because of the lack of sensitivity for low-grade tumor and may result in false positives because of inflammation and hematuria [62, 61].
As liquid biopsy is considered a non-invasive and repeatable test that allows dynamic assessment of specific molecular markers, many efforts have been made to identify new circulating/urinary biomarkers including cell-free DNA (cfDNA), circulating tumor cells (CTCs), circulating microRNAs (miRNAs), and exosomes, which are capable of diagnosing diseases, monitoring recurrence, and potentially predicting treatment response. The ongoing studies on the clinical significance of liquid biopsy are summarized in Table 2.
| Reference | Trial no.† | Type of study/starting date, study design | Status of the study | Solid tumors | Intervention/treatment | Primary purpose | Estimated accrual/country | Clinical findings |
|---|---|---|---|---|---|---|---|---|
| ctDNA | ||||||||
| NA | NCT04412070 |
Observational/2020, cohort, prospective |
Not yet recruiting | BC | NA | Guide therapeutic decision | 40, France | NA |
| NA | NCT04339933 |
Observational/2020, cohort, prospective |
Not yet recruiting | BC | Diagnostic test: FGFR test | Predict treatment efficacy | 92, Korea | NA |
| NA | NCT03837821 |
Interventional/2019, single group assignment |
Recruiting | BC | Drug: abemaciclib | Predict treatment efficacy | 20, US | NA |
| Park et al. [115] | NCT04197414 |
Observational/2019, cohort, prospective |
Recruiting |
BC, other solid cancers‡ |
NA |
Differential diagnosis, monitor disease progression, predict treatment efficacy. |
3000, Korean | SETD2 and DDX11 mRNA can serve as non-invasive plasma biomarkers for predicting high-grade ccRCCs (AUC=0.971). |
| NA | NCT04138628 |
Interventional/2019, non-randomized, single group |
Recruiting |
BC, metastatic BC |
Drug: atezolizumab |
Monitor disease progression, predict treatment efficacy. |
282, Denmark | NA |
| Rijnders et al. [116] | NCT03263039 |
Interventional/2017, single group assignment |
Recruiting | TCC BC | Drug: pembrolizumab | Guide therapeutic decision | 80, Netherlands | The decrease of peripheral CD4 T cells expressing chemokine receptors is an early response marker during pembrolizumab treatment in mUC. |
| NA | NCT02546661 |
Interventional/2016, randomized, parallel assignment |
Active, not recruiting | MIBC |
|
Predict treatment efficacy | 156, US | NA |
| NA | NCT03517332 |
Observational/2015, cohort, prospective |
Unknow |
BC, other solid cancers§ |
Diagnostic test: multiplexed primer and probe design developed | Assess process feasibility | 10000, US | NA |
| CTCs | ||||||||
| NA | NCT04280640 |
Observational/2020, cohort, prospective |
Not yet recruiting |
BC other solid cancers¶ |
Other: blood draw | Predict treatment efficacy | 40, US | NA |
| NA | NCT04358718 |
Interventional/2020, randomized, parallel assignment |
Recruiting | BC |
|
Monitor disease progression | 58, China | NA |
| NA | NCT02716961 |
Interventional/2016, randomized, parallel assignment |
Recruiting | Moderate-high risk NMIBC | Drug: gemcitabine, cisplatin | Predict treatment efficacy | 208, China | NA |
| NA | NCT02080650 |
Interventional/2014, non-randomized, single group assignment |
Completed |
BC, other solid cancers£ |
|
Assess device feasibility | 62, US | NA |
| Choueiri et al. [117] | NCT01780545 |
Interventional/2013, randomized, parallel assignment |
Completed |
BC, UC |
|
Predict treatment efficacy | 200, US | In the platinum-pretreated population of advanced UC, adding OGX-427 to Docetaxel provided a statistically significant improvement in OS. |
| Bellmunt et al. [118] | NCT01454089 |
Interventional/October 2011, randomized, parallel assignment |
Completed |
Metastatic BC, urologic neoplasms, urinary tract neoplasms |
|
Predict treatment efficacy | 183, US | Advanced BC patients with poor prognosis benefited from apatorsen 600mg combined with first line GC. Apatorsen may be impacting the intrinsic biology of patients with poor risk factors. |
| NA | NCT00829920 |
Observational/2008, cohort, prospective |
Completed | BC | NA | Predict treatment efficacy | 44, US | NA |
| NA | NCT02345473 |
Observational/2005, cohort, prospective |
Completed | BC | Genetic: detection of circulating tumor cells in blood samples | Liquid biopsy characterization | 59, Italy | NA |
| Cell-free RNA | ||||||||
| Abdelgawad et al. [119] | NCT03591367 |
Interventional/2018, single group assignment |
Completed | NMIBC |
|
Differential diagnosis | 115, Egypt | As molecular urinary biomarkers: E2F3 (AUC=0.889) and hTERT (AUC=0.872) have the highest potential for prediction of the grade of NMIBC to either low or high grade. |
| Exosome | ||||||||
| NA | NCT04155359 |
Observational/2020, cohort, prospective |
Recruiting | BC | NA | Differential diagnosis | 3000, US | NA |
- Abbreviations: BC, bladder cancer; ctDNA, circulating tumor DNA; ccRCCs, clear cell renal cell carcinoma; FGFR, fibroblast-growth factor receptor; SETD2, SET domain-containing 2; DDX11, DEAD/H-box helicase 11; TCC BC, transitional cell carcinoma of the bladder; mUC, metastatic urological cancer; CTCs, circulating tumor cells; EpCAM, epithelial cell adhesion molecule; UC, urological cancer; OS, overall survival; GC, gemcitabine and cisplatin; NMIBC, non-muscle-invasive bladder cancer; hTERT, human telomerase reverse transcriptase; E2F3, E2F transcription factor 3; AUC, area under curve; NA, not available.
- † http://clinicaltrials.gov/.
- ‡ Prostate cancer, renal cell cancer, ureter cancer.
- § Colorectal cancer, pancreatic adenocarcinoma, gastric cancer, hepatocellular carcinoma, non-small cell lung cancer, melanoma, ovarian cancer, adrenocortical cancer, breast cancer.
- ¶ Metastasis lung, metastasis to liver, gastrointestinal Cancer.
- £ Prostate cancer, renal cell carcinoma, colorectal cancer, gastric cancer, pancreatic cancer, non-small cell lung cancer, advanced MET amplified solid tumor.
3.1 cfDNA
Substantial evidence has demonstrated that cfDNA originated from tumor cells contains tumor-specific DNA alterations, generally referred as circulating tumor DNA (ctDNA), plasma tumor DNA, or urinary cell-free DNA (UcfDNA), which can be detected in the blood or urine of patients with BC [63-69].
Hypermethylation of the CpG island of promoter regions causes tumor suppressor gene silencing, and it has been reported for numerous gene sites in BC, such as hypermethylation at APC regulator of WNT signaling pathway (APC) [70], glutathione s-transferase pi 1 (GSTP1) [71], prostaglandin-endoperoxide synthase 2 (PTGS2) [72], and Reprimo (RPRM) [73]. However, the gene sites mentioned above were performed single gene analysis, and sensitivity was limited at 18% to 48% [66]. Using a methylation-specific PCR, Ellinger et al. [66] detected hypermethylation in at least one of the 3 genes (GSTP1, tazarotene-induced gene 1 (TIG1), and APC) in 80% of serum samples from BC patients undergoing cystectomy, with a specificity of 93% (Table 3). Furthermore, they investigated two small DNA fragments (124 bp PTGS2 and 271 bp RPRM in serum DNA levels using the same cohorts, and an apoptosis index (ratio of 124 bp/271 bp fragments) has been identified to discriminate BC from benign prostate hyperplasia with high sensitivity (96%) and moderate specificity (62%) [68] (Table 3).
| Reference | Biomarkers | Patients | Controls | Molecular targets | Method | Sample type | Sensitivity (%) | Specificity (%) | Clinical application |
|---|---|---|---|---|---|---|---|---|---|
| Vandekerkhove et al. [3] | ctDNA | 51 MIBC (37 with metastatic disease) | None | 50 BC driver genes | Targeted sequencing | Plasma | NA | NA | Prognosis |
| Birkenkamp-Demtroder et al. [65] | ctDNA | 26 MIBC | None | 84 personalized assays targeting 61 genes | Tumor-specific ddPCR assays | Plasma | NA | NA | Predict recurrence |
| Ellinger et al. [66] | cfDNA | 45 BC | 45 BPH | GSTP1, TIG1, APC | Methylation- specific PCR | Serum | 93.0 | 80.0 | Diagnosis |
| Ellinger et al. [68] | cfDNA | 45 BC | 45 BPH | PTGS2/RPRM ratios | qRT-PCR | Serum | 96.0 | 62.0 | Diagnosis |
| Patel et al. [69] | cfDNA, UcfDNA | 17 MIBC | None | 8 BC common mutated genes | TAm-Seq, sWGS | Plasma | NA | NA | Predict recurrence, predict treatment response |
| Urine supernatants | NA | NA | |||||||
| UCP | NA | NA | |||||||
| Xu et al. [76] | UcfDNA | 189 BC | 166 hematuria | IQGAP3/BMP4 and IQGAP3/FAM107A ratios | RT-PCR | Urine supernatants | 71.0 | 88.6 | Diagnosis |
| Stasik et al. [78] | UcfDNA | 53 BC | 36 HCs | Two abundant point-mutations (C228T/C250T) in the TERT promoter | NGS | Urine supernatants | 63.0 | 100.0 | Diagnosis |
| Urine sediment | 77.0 | 97.0 | |||||||
| Springer et al. [79] | Urinary cell DNA | 570 patients at risk for BC | 188 HCs | 11 UC common mutated genes | UroSEEK† | UCP | 83.0 | 93.0 | Diagnosis |
| Springer et al. [79] | Urinary cell DNA | 322 BC after surgery | 188 HCs | 11 UC common mutated genes | UroSEEK† | UCP | 68.0 | 80.0 | Predict recurrence |
| Ward et al. [80] | Urinary cell DNA | 120 early-stage BC | 20 no-cancer controls, 89 cancer-free NMIBC | 6 BC associated genes | Multiplex PCR, NGS | UCP | 70.0 | 97.0 | Diagnosis |
| Hirotsu et al. [81] | cfDNA, UcfDNA | 25 NMIBC | 5 cystitis and benign tumor | 71 UC common mutated genes | Targeted sequencing | Plasma | NA | NA | Diagnosis |
| Urine supernatants | 67.0 | NA | |||||||
| Urine sediment | 78.0 | NA | |||||||
| Dudley et al. [84] | UcfDNA | 118 early-stage BC | 67 HCs | 460-gene sequencing panel | CAPP-Seq | Urine supernatants | 93.0 | 96.0 | Diagnosis |
| Qi et al. [86] | CTCs | 57 BC | 48 HCs, 15 benign urologic pathologies | Folate receptor α | Ligand-targeted PCR | Whole blood | 82.1 | 61.9 | Diagnosis |
| Zhang et al. [97] | Exosome | 260 BC | 260 HCs | lncRNA: PCAT-1, UBC1, SNHG16 | qRT-PCR | Serum | 80.0-85.0 | 75.0-78.0 | Diagnosis |
| Zhan et al. [98] | Exosome | 184 BC | 184 HCs | lncRNA: MALAT1, PCAT-1, SPRY4-IT1 | qRT-PCR | Urine | 62.5-70.2 | 85.0-85.6 | Diagnosis |
| Armstrong et al. [100] | Exosome | 85 BC | 45 HCs | miR-21, miR-93, miR-200c, miR-940 | qRT-PCR | Urine | 88.0 | 78.0 | Diagnosis |
| Long et al. [101] | Exosome | 34 BC | 9 HCs | miR-375, miR-146a | qRT-PCR | Urine | - | - | Diagnosis |
| Murakami et al. [102] | Exosome | 173 BC | 36 no-cancer controls | mRNA: SLC2A1, GPRC5A, KRT17 | qRT-PCR | Urine | SLC2A1: 64.0 | SLC2A1: 75.0 | Diagnosis |
| GPRC5A: 54.0 | GPRC5A: 72.0 | ||||||||
| KRT17: 68.0 | KRT17: 58.0 | ||||||||
| Lin et al. [103] | Exosome | 129 BC | 62 HCs | protein: α1-antitrypsin, H2B1K | MALDI-TOF | Urine | 62.7 | 87.6 | Diagnosis |
| Chen et al. [104] | Exosome | 28 BC | 12 hematuria | protein: TACSTD2 | LC-MRM/MS | Urine | 65.0 | 75.6 | Diagnosis |
| Du et al. [120] | Exosome | 230 BC | 230 HCs | lncRNA: uc004cox.4, GAS5 | qRT-PCR | Urine | 80.0-84.5 | 78.2-85.0 | Diagnosis |
- Abbreviations: BC, bladder cancer; ctDNA, circulating tumor DNA; cfDNA, cell-free DNA; UcfDNA, urine cell-free tumor DNA; BPH, benign prostate hyperplasia; PTGS2, prostaglandin-endoperoxide synthase 2; RPRM, reprimo; qRT-PCR, quantitative real-time PCR; GSTP1, glutathione s-transferase pi 1; TIG1, tazarotene-induced gene 1; APC, APC regulator of WNT signaling pathway; ddPCR, droplet digital PCR assay; MIBC, muscle-invasive bladder cancer; TAm-Seq, tagged-amplicon sequencing; sWGS, shallow whole genome sequencing; UCP, urine cell pellet; NMIBC, non-muscle invasive bladder cancer; UC, urological cancer; HCs, healthy controls; IQGAP3, IQ motif containing GTPase activating protein 3; BMP4, bone morphogenetic protein 4; FAM107A, IQGAP3/family with sequence similarity 107A; CAPP-Seq, cancer personalized profiling by deep sequencing; NGS, next-generation sequencing technology; PCAT-1, prostate cancer associated transcript 1; UBC1, upregulated in bladder cancer 1; SNHG16, small nucleolar RNA host gene 16; MALAT1, metastasis associated lung adenocarcinoma transcript 1; SPRY4-IT1, sprouty receptor tyrosine kinase signalling antagonist 4-intronic transcript 1; GAS5, growth arrest specific 5; SLC2A1, solute carrier family 2 member 1; GPRC5A, G protein-coupled receptor class C group 5 member A; KRT17, keratin 17; H2B1K, histone H2B type 1-K; MALDI-TOF, matrix-assisted laser desorption ionization time-of-flight; TACSTD2, tumor-associated calcium signal transducer 2; LC-MS/MS, isotopic demethylation labeling coupled with liquid chromatography-tandem mass spectrometry; NA, not available.
- † UroSEEK: a massively parallel sequencing-based assay.
With the wide application of NGS technology, noninvasive testing of ctDNA in BC has been used to identify genomic aberrations and predict treatment response. For example, alterations in BRCA1 DNA repair associated (BRCA1) and Raf-1 proto-oncogene (RAF1) genes appear to be negatively associated with clinical outcomes [63], and the sequencing results revealed high genomic concordance between the tissue DNA and ctDNA. Moreover, changes in ctDNA variant allele frequencies are closely correlated with duration of immunotherapy, antitumor activity, and clinical outcomes in BC [74]. A phase I trial conducted by Sundahl et al. [64] showed a rapid ctDNA fraction decline in patients who responded to treatment, whereas stable or increased fractions were detected in non-responders. This is the first trial to demonstrate that ctDNA fraction monitoring can be used to predict treatment response for metastatic urothelial carcinoma. Similarly, Birkenkamp-Demtroder et al. [65] observed that ctDNA levels of patients with metastatic relapse were significantly higher than those of patients without cancer. The median positive interval between ctDNA detection in plasma and diagnosis of relapse was 101 days after cystectomy, suggesting that ctDNA analysis may be more sensitive than computed tomography (CT) scanning in MIBC.
Besides ctDNA, urothelial tumors have close contact with urine, thus, urinary biomarkers are highly promising as noninvasive tools. Somatic mutations are reliably detected in urinary cell-pellet DNA and cfDNA [75-80], and the level of cfDNA in urine supernatants was associated with pathologic features and disease progression, which makes urine tumor DNA have great potential in clinical detection, especially in the early stages of BC [81, 82, 80, 79, 83, 84]. Current research on the clinical significance of UcfDNA detection for BC is increasing (Table 2).
Christensen et al. [83] found that NMIBC patients had high levels of cfDNA in serial urine supernatants, which was associated with later disease progression in NMIBC. Increased levels of FGFR3 and Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) mutated DNA in urine and plasma are indicative of later progression and metastasis in BC. Hirotsu et al.[81] compared detection rates of 71 UC commonly mutated genes in urine supernatants, urine precipitation, and plasma from 25 BC patients and 5 patients with cystitis and benign tumor. The diagnostic sensitivity of the genetic analysis was higher in urine DNA (67%-78%) compared to cfDNA or conventional cytology (22%) in NMIBC. In addition, by using a high-throughput sequencing-based hybrid capture method, Dudley et al. [84] observed a high concordance for mutations between tumor and ucfDNA which enabled genotyping of multiple somatic aberration types across a broad genomic space in a single integrated assay, and they concluded that profiling of urine supernatants could have a significant value for BC early detection, sensitivity and specificity was achieved at 93% and 96% respectively (Table 3).
3.2 CTCs
Although inter-tumoral heterogeneity, as reflected in different clinical phenotypes in different patients, has been partially explained with cfDNA, the cfDNA analysis cannot fully explain ITH because of its limited origin and accurate calling of copy-number aberrations [24].
Single-cell technologies now allow a comprehensive analysis of tumor cells in peripheral blood to unravel diverse aspects of metastasis, which can contribute to tumor heterogeneity. In some cases, it may be easier to distinguish tumor heterogeneity from CTCs than from the primary tumors. Recent molecular and clinical studies have shown that invasion may occur early in tumor development, and CTCs are released into circulation in the early stage of cancer [85]. Qi et al. [86] explored an effective strategy for enrichment, characterization, and quantification of CTCs based on the high expression of folate receptor α (FRα) in BC, which validated its diagnostic significance and demonstrated a modest sensitivity of 82.1% and specificity of 61.9%. This is so far the first study to confirm that FRα can be used as a tumor marker to detect CTCs in BC. New methods for isolating large numbers of CTCs are still being studied, which may improve the ability to perform a more comprehensive molecular analysis of most patients.
Currently, a preliminary study comparing CTC and ctDNA sample collection in 16 metastatic UC patients has found a similar detection rate for CTCs and ctDNA. However, the CTCs count was not correlated with cell-free DNA or ctDNA fraction, and several clinically actionable mutations were detected in plasma that were not found in the matching tumor [87]. Therefore, the researchers suggested that CTCs and ctDNA can provide complementary information; ctDNA may be more effective for early detection, while CTCs may be more suitable for studying the biological features of circulating malignant cells as well as protein expression at the single cell level. The presence of CTCs has also been proposed to be associated with poor prognosis, and the amount of CTCs found in blood is often associated with short-term disease-free survival of metastatic BC [88].
3.3 Circulating miRNAs and others
Other tumor-derived products also exist in bodily fluids, such as cell-free miRNAs [60-62], long non-coding RNAs (lncRNAs) [63], exosomes or tumor-educated platelets [61], which are of concern as liquid biopsy analyses with potential clinical significance.
miR-210 was upregulated in BC tissues, and the levels of miR-210 increased with advancing stage and grade [89]. Moreover, miRNA expression levels can also reflect tumor dynamics in MIBC. Yang et al. [90] compared miR-210 levels in matched serum samples obtained before and after surgery, and the results showed a significant reduction of miR-210 after surgery. The levels of serum miR-210 from patients with relapsed BC were upregulated and reached the levels of those in pre-operative patients. In summary, these studies suggest that serum miR-210 could be a potential noninvasive biomarker for screening, predicting, and monitoring MIBC.
Exosomes are vesicles of endocytic origin and continuously released from the bladder [91], which can mediate communication between cells through the transfer of nucleic acids [92-95] and proteins [96] in BC. Numerous studies have been conducted to investigate the role of exosomes in patients with BC. The results are very heterogeneous, but promising candidate nucleic acids biomarkers have been identified with higher sensitivities and specificities than plasma ctDNA. The major studies that have investigated the role of exosomes in the diagnosis of BC are compiled in Table 3 [86, 97-104]. Although the specific functional role of exosomes in BC heterogeneity remains unclear, exosomes have emerged as potential noninvasive disease biomarkers with potential clinical significance.
4 CONCLUSIONS AND FUTURE PERSPECTIVES
BC is a heterogeneous disease with higher tumor burden among cancers. Unfortunately, there have been few advances in its clinical management due to a poor understanding of the correlations between its molecular and clinical features. This tumor heterogeneity might ultimately provide the fuel for the emergence of treatment resistance and, eventually, disease relapse.
An alternative strategy to understand tumor heterogeneity might be liquid biopsy, which can detect genetic alterations and tumor cells present in peripheral blood or urine of patients with BC [105, 99]. Peripheral blood- or urine-based genomic analyses were flexible in processing, and there are no specific device required for isolation, which have the potential to diagnose and dynamically monitor BC recurrence [82, 78, 106, 69, 65, 79]. Therefore, cfDNA analysis has now become one of the major methods to evaluate BC heterogeneity without the sampling bias of tissue biopsy. However, there are still remaining challenges of widely used cfDNA. At present, majority of studies on liquid biopsy in BC are based on ctDNA analysis and focus on patients at advanced stages. Since the use of ctDNA for early detection is probably very limited by the low detection rates in early-stage cancer, highly sensitive methods and sufficient sample volumes are needed to detect trace amounts of ctDNA [107, 108].
Compared to the peripheral blood collection, urine biopsy can be truly collected “non-invasively”, and sampling peripheral fluids in close proximity to diseased organs has been proved to improve the sensitivity of the detection of tumor mutated DNA [109, 69]. Therefore, urine-based genetic analysis is an ideal liquid biopsy for detecting tumor-derived DNA and may be more precise in reflecting tumor mutational profiles than plasma, especially in early-stage BCs [81, 69].
Attention should also be paid to the effects of confounders of apoptosis and aging on cfDNA analysis in non-tumor patients. For example, benign somatic heterogeneity may result from somatic mosaicism, which is the accumulation of mutations during development and aging, resulting in the production of cells with different genotypes in the same individual. Fernandez-Cuesta et al. [110] assessed the presence of tumor protein p53 (TP53) mutations detected at very low fractions in the cfDNA, and they detected TP53 mutations in 49% small cell lung cancer patients and 11.4% of non-cancer controls. Therefore, detection of a mutation in a cancer driver gene in cfDNA cannot be equated with evidence of tumor.
In addition to genomic aberrations not covered by ctDNA analysis, CTC analysis provides unique insights into tumor heterogeneity, including transcriptional heterogeneity using single-cell RNA sequencing [111]. However, there is very low number of CTCs available in early stage disease, and different tumor regions within an individual patient might not present the same propensity of CTCs. Therefore, it is still unclear whether CTCs analysis could lead to a potential biological bias with the ITH. In the near future, besides the development of novel high-throughput technologies, the combined molecular characterization of ctDNA and CTCs or other liquid biopsy may provide complementary information, for example, combining ctDNA- and CTC-derived analyses can identify the T790M mutation in EGFR-mutant non-small cell lung cancer patients in whom the concurrent biopsy is negative or uncertain [112].
Other tumor products such as cell-free RNA or exosomes also have high sensitivity and specificity in the diagnosis of BC, whereas as exosomes can be released into the peripheral circulation by many types of cells, the key challenges is to discriminate exosomes derived from either tumor or normal cells.
Recent advances in detection and sequencing technology have contributed to clinical determination of genomic heterogeneity in subpopulations of BC patients. For example, the detection of invasive mesenchymal exudative bladder cancer cells (EBCCs) from urine using a microfluidic enrichment device has a high sensitivity (93.3%), therefore, the enumeration and cytological analysis of EBCCs may serve as a complementary tool for clinical real-time detection [113]. In nasopharyngeal cancer, a label-free and modification-free nanotechnology based on surface-enhanced Raman spectroscopy was employed for cfDNA analysis with an ideal diagnostic sensitivity of 83.3% and specificity of 82.5% [114]. These advancements have increased the sensitivity of non-invasive screening for early diagnosis. In addition, the extent of genomic and transcriptional heterogeneity at the individual cell level remains largely unknown within primary bladder tumors and metastases. Large-scale clinical trials involving various stages of BC will be needed to determine the clinical value of spatial and temporal variation in the genomic and transcriptional landscape of BC to guide clinical treatment.
DECLARATIONS
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
COMPETING INTERESTS
The authors declare that they have no competing interests.
FUNDING
This work was supported by grants from the National Natural Science Foundation of China [82072369].
AUTHORS' CONTRIBUTIONS
Conception: all authors. Manuscript writing: HM Huang. Manuscript revision: HX Li. All authors read and approved the final manuscript.
ACKNOWLEDGEMENTS
We thank Professor Wei Yu and Han Hao for their valuable scientific advice and discussion.




