Lung adenocarcinoma patients with novel ALK fusion variants and their clinical responses to ALK inhibitors
Abbreviations
-
- ALK
-
- anaplastic lymphoma kinase
-
- ALK-TKIs
-
- ALK tyrosine kinase inhibitors
-
- ALT
-
- alanine aminotransferase
-
- AST
-
- aspartate aminotransferase
-
- C11orf30
-
- chromosome 11 open reading frame 30
-
- CH
-
- calponin-homology
-
- CK7
-
- cytokeratin 7
-
- CSF
-
- cerebrospinal fluid
-
- CT
-
- computed tomography
-
- ctDNA
-
- circulating tumor DNA
-
- CTCAE
-
- common terminology criteria for adverse events
-
- ECT
-
- emission computed tomography
-
- EML4
-
- echinoderm microtubule-associated protein-like 4
-
- HE
-
- hematoxylin and eosin
-
- IGR
-
- intergenic region
-
- MRI
-
- magnetic resonance imaging
-
- NCCN
-
- National Comprehensive Cancer Network
-
- NGS
-
- next-generation sequencing
-
- NSCLC
-
- non-small cell lung cancer
-
- PKHD1
-
- polycystic kidney and hepatic disease 1
-
- RECIST
-
- response evaluation criteria in solid tumors
-
- TTF-1
-
- thyroid transcription factor-1
Dear Editor,
Lung cancer is one of the most common cancers worldwide and is associated with high mortality. Anaplastic lymphoma kinase (ALK) rearrangement, an oncogenic driver, has been identified in 5% to 6% of patients with non-small cell lung cancer (NSCLC) [1]. The first identified and the most common ALK fusion partner is echinoderm microtubule-associated protein-like 4 (EML4) [2]. With the broad application of next-generation sequencing (NGS), an increasing number of novel ALK fusions have been reported. Many ALK tyrosine kinase inhibitors (ALK-TKIs), including crizotinib, brigatinib, ceritinib, and ensartinib, have been approved to treat ALK-positive NSCLC patients, and their efficacy may be affected by different ALK fusion variants [3]. Here, we report two novel ALK fusions, MAPRE3-ALK and PKNOX2-ALK, detected by an NGS panel targeting 425 cancer-related genes (Nanjing Geneseeq Technology Inc., Nanjing, Jiangsu, China) [4] in two metastatic lung adenocarcinoma patients who then received ALK-TKI treatments. The drug efficacy of the two novel fusions reported here could have significant referential value and provide useful therapeutic strategies for treating ALK-positive patients.
Case number 1 was a 38-year-old female who was referred to the Second Hospital of Dalian Medical University (Dalian, China) in December 2018 after a routine physical examination. Her chest computed tomography (CT) scan revealed a solitary mass (45 mm × 32 mm) in the lower-right pulmonary lobe and a small nodule in the left lobe (Figure 1A). Hematoxylin and eosin (HE) staining of CT-guided fine-needle aspiration cytology of the lower-right solitary mass revealed a typical adenocarcinoma histology with positive immunohistochemistry markers including thyroid transcription factor-1 (TTF-1), NapsinA and cytokeratin 7 (CK7), and negative P40 expression. In addition, she exhibited enlarged lymph nodes in the right hilum, suggesting lymph node metastases. Subsequent targeted NGS analysis of her CT-guided aspiration biopsy identified a MAPRE3-ALK fusion (Figure 1B), but no other oncogenic alterations were found (Supplementary Table 1). Therefore, the patient was prescribed crizotinib on February 3, 2019 (250 mg, twice daily via oral administration) as the first-line therapy and achieved a partial response (all treatment responses mentioned were assessed based on Response Evaluation Criteria In Solid Tumors [RECIST] version 1.1), as indicated by chest CT scan re-examination one month later which showed a remarkable shrinkage of the tumor (26 mm × 23 mm, Figure 1A). However, the patient experienced fatigue, nausea, and grade 4 hepatic impairment, as demonstrated by a significant increase in alanine aminotransferase (ALT, 3843.24 U/L) and aspartate aminotransferase (AST, 1553.91 U/L), according to the Common Terminology Criteria for Adverse Events (CTCAE).
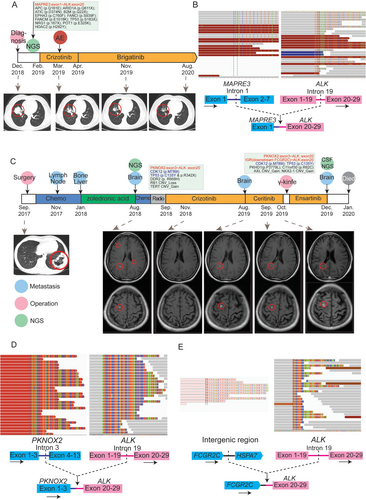
Schematic of patients’ treatment histories and NGS-detected gene rearrangements. (A) Chronological presentation of the clinical history and medical treatment for Case 1. The mutations detected by NGS are listed, with the anaplastic lymphoma kinase (ALK) fusion highlighted in red. Images of the chest CT are provided with lung tumors indicated with red circles. (B) NGS reads in the two MAPRE3-ALK fusion breakpoint regions are visualized using the Integrated Genomics Viewer (Broad Institute, Cambridge, MA, United States). The schematic below shows the fused exons of the MAPRE3-ALK rearrangement. The mean on-site coverage of the sample was 950× and the coverage of the MAPRE3-ALK fusion was 1222×. (C) Chronological presentation of the clinical history and medical treatment for Case 2. The common mutations in the primary lung tumor and cerebrospinal fluid (CSF) circulating tumor DNA (ctDNA) are shown in blue, and ALK fusions are shown in red. Pink circles indicate the clinical operations (diagnosis, lung surgery, and brain γ-knife), blue circles indicate metastatic sites, and green circles show the time point of NGS. Images of the chest CT and brain MRI at two layers are provided, with the primary lung tumor and intracranial metastases indicated by the red circles. (D-E) NGS reads in the two ALK fusion breakpoint regions are visualized using the Integrated Genomics Viewer (Broad Institute). The schematic below shows the PKNOX2-ALK and IGR-ALK rearrangements. The mean on-site coverage of PKNOX2-ALK and IGR-ALK was 774× and 415×, respectively. The detected fusion coverage was 826× and 338×.
Abbreviations: NGS: next-generation sequencing; CT: computed tomography; MRI: magnetic resonance imaging; IGR: intergenic region; ALK: anaplastic lymphoma kinase; CSF: cerebrospinal fluid; ctDNA: circulating tumor DNA
Due to the severe adverse effects from crizotinib, the patient was then switched to brigatinib (160 mg per day via oral administration) in April 2019. A follow-up chest CT scan showed a partial response to brigatinib (lesion size: 20 mm × 16 mm) and the most recent follow-up examination in August 2020 revealed continuous shrinkage of the tumor (17 mm × 12 mm). This case demonstrated that the MAPRE3-ALK fusion conferred sensitivity to ALK-TKIs, including crizotinib and brigatinib, with brigatinib being more tolerable.
Case number 2 was a 48-year-old female with a solitary mass (89 mm × 56 mm) in the lower-left pulmonary lobe (Figure 1C) identified by chest CT on August 31, 2017. The mass was surgically removed in September 2017. The postoperative pathology revealed a moderate-to-low differentiated stage IIIB lung adenocarcinoma with pleural invasion and mediastinal lymph node metastases. The patient initially received two cycles of a TP regimen (240 mg of docetaxel via intravenous guttae on day 1 and 40 mg of cisplatin via intravenous guttae on days 1-3; repeated every 21 days), but still developed recurrence in her right cervical lymph nodes (largest size: 2.5 cm × 1.1 cm). According to the National Comprehensive Cancer Network (NCCN) guidelines, two cycles of pemetrexed (0.8 g; intravenous guttae on day 1) plus carboplatin (0.5 g; intravenous guttae on day 1) and bevacizumab (400 mg; intravenous guttae on day 1, repeated every 21 days) were administered and led to the shrinkage of metastatic sites (largest size: 0.8 cm × 0.6 cm) in April 2018. The tumors remained stable during all follow-up cervical ultrasonography performed over a two-month interval until June 2019. However, bone metastasis was later found by bone emission computed tomography (ECT) as the patient reported occasional osphyalgia. As of January 2018, the bone metastasis was treated by regular anti-bone metastasis therapy using zoledronic acid (4 mg via intravenous guttae; repeated every 28 days), according to NCCN guidelines.
In August 2018, the surgically-resected tumor sample underwent targeted NGS when multiple intracranial metastases, ranging from 3 mm to 11 mm, were found by routine follow-up magnetic resonance imaging (MRI). After one cycle of chemotherapy on August 7, 2018 (0.6 g pemetrexed via intravenous guttae on day 1 and 0.35 g carboplatin via intravenous guttae on day 2; repeated every 21 days) and craniocerebral radiotherapy (2 Gy/fraction, 22 fractions) from August 17, 2018, to September 21, 2018, the patient was prescribed crizotinib in September 2018 (250 mg, twice daily via oral administration) as NGS identified a novel PKNOX2-ALK fusion (Figure 1D). No other druggable mutations were identified by NGS (Table 1).
The brain lesions exhibited a partial response to crizotinib, as shown by follow-up MRIs (largest size: 3 mm) in November 2018. The lesions remained stable until July 2019, during which MRI examinations were performed every two months. Ceritinib (450 mg, once daily via oral administration) was administered to the patient when the brain metastases, ranging from 5 mm to 11 mm, relapsed in August 2019. MRI examinations after one month of ceritinib treatment suggested stable disease at the brain metastatic sites. However, the brain lesions progressed rapidly (lesion size: 4 mm - 12 mm) in October 2019. The patient underwent γ-knife treatment and received ensartinib (225 mg, once daily via oral administration) beginning in November 2019, but she passed away in January 2020 due to multiple relapsed brain metastases. No adverse events were reported during the treatment.
During the final hospitalization, cerebrospinal fluid (CSF) was collected for circulating tumor DNA (ctDNA) sequencing. Compared to the primary lung tumor, CSF ctDNA sequencing detected a unique intergenic region rearrangement (IGR-ALK) where ALK exon 20 fused to an intergenic region on chromosome 11, in addition to the PKNOX2-ALK fusion detected in the primary lung tumor (Figure 1E). The complete list of genetic alterations detected in the CSF sample and primary lung tumor is shown in Table 1. Some unique mutations in the CSF sample were also identified, including polycystic kidney and hepatic disease 1 (PKHD1) and chromosome 11 open reading frame 30 (C11orf30), which suggested clonal evolution of the tumor during metastasis.
The breakpoints of the MAPRE3-ALK in Case 1 were located in MAPRE3 intron 1 and ALK intron 19, which encode the MAPRE3 calponin-homology (CH) domain and the ALK tyrosine kinase domain, respectively (Figure 1B). MAPRE3 is a microtubule-associated protein that belongs to the same protein family as EML4, the first identified and most common ALK fusion partner. A case study [5] reported a similar MAPRE3-ALK fusion in colorectal adenocarcinoma with a breakpoint in MAPRE3 exon 7, but no clinical treatment information was reported.
This study illustrated the first case of a MAPRE3-ALK fusion in a lung cancer patient (Case 1) and reported that the patient had a modest clinical response to ALK-TKIs, including crizotinib and brigatinib. In addition, we also observed a novel PKNOX2-ALK fusion in a lung cancer patient (Case 2) who had no other concurrent oncogenic mutations. Of note, ALK TKIs had mixed effects on PKNOX2-ALK fusion. Whether MAPRE3-ALK or PKNOX2-ALK produces functional fusion proteins remains to be determined. In conclusion, we reported two novel ALK fusions in two lung cancer patients with modest clinical responses to ALK-TKIs. The functions of the two fusions in oncogenesis and drug resistance need to be further investigated to better inform treatment decisions and prognosis predictions. Our study provides new information for the direct treatment of ALK-positive patients and provides the first clinical evidence of ALK-TKI efficacy in such patients.
DECLARATIONS
ACKNOWLEDGMENTS
We would like to thank the patients and family members who gave their consent to present the data in this study, as well as the investigators and research staff involved in this work.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The authors hold written informed consent.
CONSENT FOR PUBLICATION
The patients provided written consent for publication of this case report.
AVAILABILITY OF DATA AND MATERIALS
Additional data and materials related to the genetic tests, pathologic reports, treatment information, and images are available for review upon request.
FUNDING
The authors received no specific funding for this work.
CONFLICT OF INTEREST
Yutong Ma, Qiuxiang Ou, and Xue Wu are full-time employees of Geneseeq Technology Inc., Toronto, Canada. The remaining authors have no conflicts of interest to declare.
AUTHORS’ CONTRIBUTIONS
DFY contributed to the study conceptualization, methodology, and wrote the original draft of the manuscript; ZXD supervised the study and edited the manuscript; DL curated and validated the data; YTM and QXO contributed to data interpretation and manuscript revision; XW interpreted the data; SQC contributed to the study administration and provided resources.




