Defensin: The immune system regulatory factor against peripheral nerve disease
Tiantian Qi and Qi Yang contributed equally to this work and shared the first authorship.
Abstract
Peripheral nerve disease is commonly encountered in orthopedics, neurology, and neurosurgery. Due to its large population, a substantial number of patients are affected by these conditions in China. Peripheral nerve disease has a high disability rate and current treatments show poor clinical efficacy, resulting in a heavy burden for patients and the country's healthcare system. Defensins are widespread proteins, commonly found in animals, plants, and fungi, with multiple subtypes able to kill a variety of pathogens. As regulatory factors of the immune system, defensins influence bodily function by participating in inflammatory processes, immune responses, and pathogen resistance; they can affect all stages of nerve conduction and play an important role in lesions of peripheral and effector nerves. This article provides a review of the possible roles and mechanisms of defensins in peripheral nerve disease.
Key points
What is already known about this topic?
-
Peripheral nerve disease can lead to sensory, motor, and autonomic dysfunction, with symptoms including pain, loss of sensation, lack of coordination, muscle weakness, and atrophy. It is a common disease in clinical practice. Defensins are important regulatory factors in the immune system of various organisms, exerting antibacterial, antiviral, and cytotoxic effects. They can help treat peripheral nerve diseases by participating in inflammatory processes, immune responses, and pathogen resistance.
What does this study add?
-
New fields in the treatment of peripheral nerve disease with defensins are reviewed, including the effects of cross-species or new subtypes of defensins, the construction of defensin derivatives, and the application of tissue engineering technology combined with defensins.
1 INTRODUCTION
Peripheral nerve disease refers to conditions that damage nerve structures in the peripheral nervous system, excluding the olfactory and optic nerves. Trauma, metabolic disorders, tumors, and genetic conditions can all lead to peripheral nerve disease. Patients with peripheral nerve injury typically experience sensory, motor, or autonomic dysfunction, with common symptoms including pain, loss of sensation, lack of coordination, muscle weakness, and atrophy. Peripheral nerve disease can lead to lifelong disability if not treated in time1 (Figure 1). Treatment strategies include surgery, medication, and physical therapy, and can typically control and alleviate symptoms. Taking nerve injury following trauma as an example, epidemiological data from the WHO show that about 5% of trauma patients develop functional limb disabilities caused by nerve damage; trauma is also the leading cause of death among people under 45 years of age. Approximately 1/4 of the global population is affected by some degree of trauma, leading to a large number of nerve injury patients. However, due to the incomplete understanding of the mechanisms of peripheral nerve disease, treatments are often unsatisfactory, resulting in a significant burden for patients and national healthcare systems.2 Advancing research on the mechanisms of peripheral nerve diseases has therefore become a key area of focus.3 For example, Zhang et al. reported that traditional methods for peripheral nerve repair are ineffective, proposing biological scaffold materials combined with seed cells and bioactive substances as a promising alternative.4 Meanwhile, Li et al. state that the development of emerging exogenous growth factors could also enhance the restorative effect of neural conduit biomaterials, providing stronger repair capabilities than traditional medications or cytokines.5 Gu et al. also proposed that the formation of a favorable regeneration microenvironment during tissue-engineered nerve transplantation would help this process replace autologous nerve transplantation and achieve effective repair.6 Besides research on novel repair materials and substances, other studies have focused on improving existing repair methods. Liao et al. compared end-to-end anastomosis and end-to-side anastomosis repair methods after peripheral nerve injury and concluded that both methods can restore nerve and effector function.7
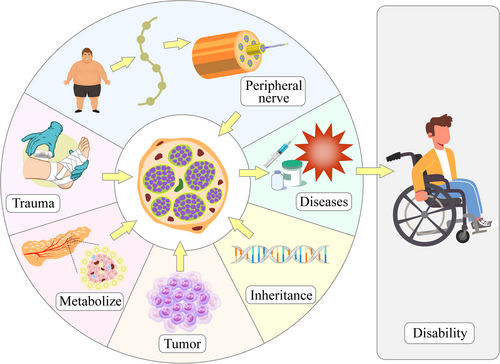
Factors leading to peripheral nerve diseases. Trauma, metabolic dysfunction, tumors, and genetic factors can all lead to peripheral nerve disease. Delayed treatment can result in disability in patients.
Defensins are found widely in different animals, plants, and fungi. They are important regulatory factors in the immune system of organisms exerting antibacterial, antiviral, and cytotoxic effects. Defensins were first discovered by Mattick et al.8 while studying bacteria and have been proven to be rich in cysteine and arginine. Further research has demonstrated that defensins can regulate the progression of neurological diseases by participating in inflammatory processes,9 immune responses,10 and pathogen resistance.11 This article provides a review of the role of defensins in peripheral nerve diseases and provides a theoretical basis for studying the pathogenesis of these disorders.
2 INTRODUCTION TO DEFENSINS
Defensins are cationic peptides rich in disulfide bonds that are capable of killing various pathogens such as bacteria. Most of these defense peptides are composed of 28–54 amino acid residues, have a molecular weight of 2–5 kDa, and contain 6–8 cysteine-based disulfide bonds.12 In mammals, defensins can be divided into three subtypes, namely α-defensins, β-defensins, and θ-defensins; insect and plant defensins also exist (Table 1; Figure 2). Most defensins are highly amphiphilic molecules formed by three-dimensional folding structures, whose ability to kill pathogens may be related to their capacity to insert into biofilms and create pores.40 In this process, defensins penetrate the microbial cell membrane, interrupting it and causing cytoplasmic leakage, ultimately leading to apoptosis.
| Subtype | Distribution | Function | Regulating diseases |
|---|---|---|---|
| α-defensin | In neutrophils, small intestinal and pancreatic cells of mammals such as humans, rabbits, and mice | Regulating immune response 13, participating in inflammatory processes 14, resisting bacteria 15, etc | Peripheral nerve injury 16, periprosthetic infection 17, osteoporosis 18, etc |
| β-defensin | In the bone marrow of cattle and epithelial cells of humans and various mammals | Participating in immune defense 19, anti-tumor 20, affecting inflammation 21, etc | Psoriasis 22, rheumatoid arthritis 23, ankylosing spondylitis 24, etc |
| θ-defensin | In the neutrophils and monocytes of mammals such as macaques, rhesus monkeys, and baboons | Antibacterial 25, antiviral 26, etc | Rheumatoid arthritis 27, acute lung injury 28, papillomavirus infection 29, etc |
| Insect defensin | Drosophila and other insects | Antibacterial 30 and antifungal 31 | Gram bacterial infection 32, Fusarium infection 33, etc |
| Plant defensin | Plants such as wheat and soybean seeds | Affecting angiogenesis 34, antifungal and bacterial 35, inhibiting cancer cell growth 36 | Leukemia 37, breast cancer 38, cadmium accumulation in plants 39, etc |
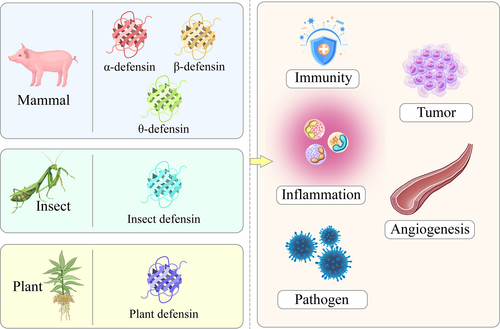
Types and functions of defensins. Defensins can be divided into mammalian defensins (α-defensins, β-defensins, and θ-defensins), insect defensins, and plant defensins. Defensins have functions such as regulating inflammation, immune responses, and angiogenesis, and exhibit pathogen-resistant and anti-tumor properties.
α-defensin is composed of an α-helix and a β-sheet and contains three disulfide bonds which are located between Cys1–Cys6 (connecting the N-terminus and C-terminus), Cys2–Cys4, and Cys3–Cys5, giving a cyclic primary structure.41 α-defensin was initially isolated from rabbit lung macrophages by the Robert Lehrer laboratory in the United States in 1980. In rabbits, α-defensin includes six subtypes, Rab1/2/3a/3b/4/5. In humans, six subtypes have been identified: hNP1/2/3/4 in the medullary system and HD5/6 in the enterogenous system (Table 2). β-defensin also contains three disulfide bonds formed by six cysteine residues located at Cys1–Cys5 and Cys3–Cys6. Its two glycines are located at the N-terminus and fold together to form three anti-parallel β-sheets and an α-helix.42 β-defensin was initially isolated from bovine tracheal epithelial cells by Diamond et al. in 1991. In humans, the main subtypes of β-defensin include HBD1/2/3/4/5/6 (Table 3); other subtypes exist in rats, mice, and other animals. θ-defensin is a small cyclic defensin,[71] with disulfide bonds located between Cys1–Cys4 and Cys3–Cys6. θ-defensin contains fewer amino acid residues and has a molecular weight of about 2 kDa. It was first discovered in 1999 as part of research on monocytes and neutrophils in rhesus monkeys. So far, only 11 subtypes have been isolated from three primate species (Table 4). The disulfide bonds of insect defensins are also located between Cys1–Cys4 and Cys3–Cys6. Insect defensin contains an amino-terminal ring, an α-helix, and a carboxyl-terminal antiparallel β-sheet.81 It was initially isolated by Hultmark et al. during their study of silkworm moths in 1980. Currently, more than 280 species have been identified. Plant defensin contains four disulfide bonds located between Cys1–Cys8 and Cys4–Cys7 and consists of an α-helix and three β-sheets.82 Plant defensin was initially isolated by Mendez et al. while studying wheat seeds in 1990.
| Subtype | Sequence | Molecular formula | Molecular weight |
|---|---|---|---|
| hNP1 | H-Ala-Cys-Tyr-Cys-Arg-Ile-Pro-Ala-Cys-Ile-Ala-Gly-Glu-Arg-Arg-Tyr-Gly-Thr-Cys-Ile-Tyr-Gln-Gly-Arg-Leu-Trp-Ala-Phe-Cys-Cys-OH | C150H222N44O38S6 | 3442.08 |
| hNP2 | H-Cys-Tyr-Cys-Arg-Ile-Pro-Ala-Cys-Ile-Ala-Gly-Glu-Arg-Arg-Tyr-Gly-Thr-Cys-Ile-Tyr-Gln-Gly-Arg-Leu-Trp-Ala-Phe-Cys-Cys-OH | C147H217N43O37S6 | 3370.95 |
| hNP3 | H-Asp-Cys-Tyr-Cys-Arg-Ile-Pro-Ala-Cys-Ile-Ala-Gly-Glu-Arg-Arg-Tyr-Gly-Thr-Cys-Ile-Tyr-Gln-Gly-Arg-Leu-Trp-Ala-Phe-Cys-Cys-OH | C151H222N44O40S6 | 3486.09 |
| hNP4 | H-Val-Cys-Ser-Cys-Arg-Leu-Val-Phe-Cys-Arg-Arg-Thr-Glu-Leu-Arg-Val-Gly-Asn-Cys-Leu-Ile-Gly-Gly-Val-Ser-Phe-Thr-Tyr-Cys-Cys-Thr-Arg-Val | C157H261N49O43S6 | 3715.44 |
| HD5 | H-Ala-Thr-Cys-Tyr-Cys-Arg-Thr-Gly-Arg-Cys-Ala-Thr-Arg-Glu-Ser-Leu-Ser-Gly-Val-Cys-Glu-Ile-Ser-Gly-Arg-Leu-Tyr-Arg-Leu-Cys-Cys-Arg-OH | C144H238N50O45O6 | 3582.18 |
| HD6 | H-Ala-Phe-Thr-Cys-His-Cys-Arg-Arg-Ser-Cys-Tyr-Ser-Thr-Glu-Tyr-Ser-Tyr-Gly-Thr-Cys-Thr-Val-Met-Gly-Ile-Asn-His-Arg-Phe-Cys-Cys-Leu-OH | C156H228N46O46S7 | 3708.27 |
| Subtype | Chromosomal location | Composition | Expression site | Regulating diseases |
|---|---|---|---|---|
| hBD1 | 8p23.2-p23.1 | Mature hBD1 is composed of 36 amino acids, with 3 disulfide bonds, 2 β-sheets, and 1 α-helix at the N-terminus | Skin 43, respiratory tract 44, kidneys 45, female reproductive tract 46, etc | Depression 47, Alzheimer's disease 47, Beckett's disease 48, vitiligo 49, bladder cancer 50, etc |
| hBD2 | 8p23.1-p22 | A cationic short peptide composed of 41 amino acid residues, with a 3-bundle layered structure and 3 disulfide bonds | Blood vessels 51, digestive tract 52, skin 53, etc | Basal cell carcinoma 54, acute graft-versus-host disease 55, necrotizing enterocolitis 56, erosive oral lichen planus 57, etc |
| hBD3 | 8p23 | A cationic short peptide composed of 45 amino acid residues, with 1 α-helix, 3 anti-parallel β-sheets, and 6 conserved cysteine residues | Epithelium or skin 58, oral cavity 59, skeletal muscle 60, tonsils 61, etc | Tuberculosis 62, gingivitis 63, pneumonia 64, asthma 65, etc |
| hBD4 | 8p23.1-p22 | A mature peptide composed of 50 amino acids, lacking an amino acid residue between the 4th and 5th cysteine residues | High expression in testes 66 and gastric antrum 66 | COVID-19 67, primary dental pulp disease 68, burns 69, gingivitis 70, etc |
| Biological effects | Signaling pathways or mechanisms that produce effects | Regulating diseases |
|---|---|---|
| Regulating metabolism | Affecting blood sugar, free fatty acids, triglycerides, and insulin | Metabolic syndrome or diabetes 72 |
| Resisting inflammation | Activating the phosphoinositide 3-kinase/protein kinase B and mitogen-activated protein kinase signaling pathways | Septicemia 73 |
| Participating in innate immunity | Activating the NF-κBsignaling pathway | Bacterial endotoxin infection 74 |
| Affecting cell proliferation | Affecting the activity of ubiquitin proteasome system | Breast cancer 75 |
| Participating in enzymatic processes | Non-competitive inhibition of protease activity or weak inhibition of invertase activity | Anthrax 76 |
| Relieving cystic fibrosis | Reducing metalloproteinase activity and secretion of inflammatory factors | Pulmonary fibrosis 77 |
| Killing fungi | Maintaining the homeostasis of neutrophil and monocyte numbers through host directed mechanisms | Invasive Candida infection 78 |
| Resisting viruses | Blocking viral DNA replication and downregulating CXCR4 | Acquired immunodeficiency syndrome 79 |
| Relieving tissue pathological changes | Immune influence on tissue cytokine response | Acute respiratory syndrome 80 |
In the following section, we will elaborate on the functions of defensins in the body and their role in peripheral nerve diseases.
3 THE ROLE OF DEFENSINS IN REGULATING BODY FUNCTIONS
Inflammation, immunity, and pathogenic infection are all dominant factors influencing the onset and course of peripheral nerve diseases.83-85 Defensins can regulate processes related to each of these factors. They also participate in functional changes in the body, further affecting the occurrence and development of peripheral nerve conditions. Understanding the pathological and physiological roles of defensins in these three processes is beneficial for the potential application of defensins in the targeted treatment of peripheral nerve diseases.
3.1 Defensins regulate the inflammatory process and affect body functions
Whole-body ionizing irradiation of adult mice has various effects, including the reduction of the expression of α-defensin in intestinal Paneth cells, depletion of α-defensin in the intestinal cavity, disruption of gut microbiota, increased permeability of the intestinal wall, and increased levels of endotoxins. Prophylactic addition of α-defensin subtype-5 to the diet of mice 24 h before irradiation prevented these processes, confirming the involvement of α-defensin in intestinal mucosal damage, endotoxemia, and systemic inflammation.86 To investigate psoriasis, a persistent inflammatory skin disease, researchers bred genetically engineered mice with a keratinocyte proline-rich protein (KPRP) modification and induced inflammation using imiquimod. Compared with wild-type mice, the KPRP heterozygous knockout inflammatory model mice exhibited reduced expression of β-defensin and increased expression of lysophosphatidic acid receptor 1, resulting in fewer γδ low T cells producing interleukin-17 (IL-17) and intensified epidermal proliferation.87 Krishnan et al. designed Pro9-3, a peptide with antibacterial activity and cytotoxicity based on insect defensin. To improve its function, they added arginine and its D-enantiomer at the N-terminus to form a new peptide antibiotic, Pro10-1D. This modified version can inhibit lipopolysaccharide (LPS)-induced macrophage inflammation through the Toll-like receptor 4 (TLR4)/nuclear factor-κB (NF-κB) signaling pathway, reduce multiple organ injury in septic mice, and alleviate systemic infection-related inflammation.88 Some researchers have also studied plant defensins and found that the pea plant defensin Psd1 can target the nucleus of cutaneous melanoma B16F10 cells in mice, leading to nuclear fragmentation and induction of the cells into the G0/G1 subphase. 1 mg/kg Psd1 significantly reduced the number of inflammatory cells in the lungs of a mouse model of metastatic lung melanoma without causing weight changes or death. This further indicates that Psd1 exhibits a cytotoxic effect limited to local inflammatory cells rather than systemic cytotoxicity.89
Different types of defensins can act on inflammatory cells, inflammatory mediators, and inflammatory signaling pathways in different diseases. Their effects can inhibit inflammatory processes at the local, tissue, organ, or systemic level. Reports on these effects provide insights for future research on the regulation of inflammatory processes in peripheral nerve diseases by defensins (Figure 3).
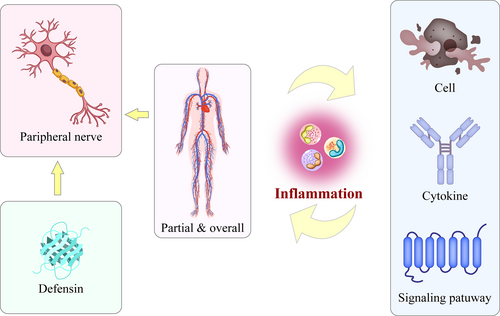
Defensins influence peripheral nerve diseases by regulating systemic and local inflammatory processes. Defensins may participate in systemic and local inflammatory processes by affecting inflammatory cells, mediators, and signaling pathways. Through such interactions, they affect the onset and development of peripheral nervous system diseases.
3.2 Defensins interfere with immune response and affect body functions
Immune cell infiltration is closely related to tissue destruction. Analysis of genetic data related to periodontitis in public databases revealed that the expression of the β-defensins HBD2 and HBD3 was reduced in gingival specimens of periodontitis. Samples with low HBD2 expression had reduced infiltration of monocytes, macrophages, and CD4+T cells, while those with low HBD3 expression had reduced infiltration of γδ T cells. Vitamin D3 stimulation increased the expression of HBD2 and HBD3 in a time-dependent manner, indicating that β-defensin is related to immune infiltration in periodontitis and is regulated by vitamin D3.90 In contrast, α-defensin is associated with innate immunity to COVID-19. In the plasma samples of COVID-19 patients, levels of α-defensin-1 (DEFA1) and peripheral blood mononuclear cells were elevated, correlating with the severity and stage of COVID-19. Cell experiments confirmed that DEFA1 secreted by immune cells inhibited the infection of original and mutant COVID-19 in a dose-dependent manner.91 Plant defensins can also strongly influence human immune regulation. Finkina et al. isolated a plant defensin, Lc-def, from lentils and found that 25–50 μM had inhibitory effects on Candida albicans, Candida krusei, and Candida smooth, but was not toxic to human cells. Lc-def was also resistant to both hydrolysis by C. albicans and cleavage by enzymes in the human gastrointestinal tract. Lc-def increases the secretion of immune cytokines required to resist Candida infection and produces pro-inflammatory cytokines due to its antifungal activity, high structural stability, and ability to activate protective immune responses.92 Researchers have also identified a potential mutual immune regulation between defensin A and a previously isolated nicotine-degrading Burkholderia cepacia with antifungal properties, providing new insights for potential nicotine-based pest control methods.93
Previous studies have found that defensins play an important role in both innate and acquired immunity in various organisms, affecting immune infiltration and influencing the onset and development of diseases. We believe that the ability of defensins to regulate immune infiltration is universal across various tissues, and does not have neural specificity. The immune-targeting effects of defensins in neural tissue may share common targets with other tissues, which may provide a foundation for future research. Moreover, different types of defensins can exert cross-species regulatory effects on immune function while exhibiting relatively little cytotoxicity on the normal cells of the affected species. This lays the foundation for cross-species research and the development of precise therapeutic defensins for peripheral nerve diseases (Figure 4).
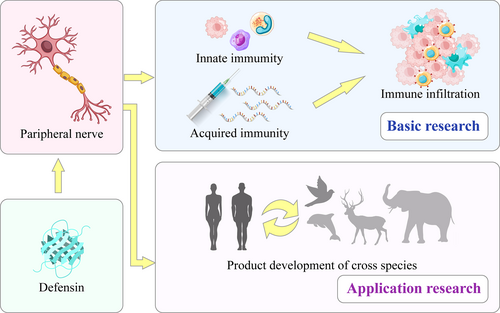
Basic and applied research on the effects of defensins on peripheral nerve diseases through intervention in innate or acquired immune responses. The immune infiltration and cross-species effects of defensins indicate potential future applications in the treatment of peripheral nerve diseases.
3.3 Defensins resist pathogen invasion and affect body function
Defensins also have an effect on the novel coronavirus. Cho et al. developed mucosal vaccine antigens designed to neutralize virus antibodies, using the papain-like protease (PLpro) from the structural domain of non-structural protein 3 of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). They found that adding β-defensins and the M cell-targeting ligand Co1 to the vaccine improved its effectiveness against the virus.94 Leannec Rialland et al. studied antifungal defensins in crops and found that the reduced form of the tick defense compound DefMT3, known as TickCore3 (TC3) γ-core, can reduce the activity of the harmful fungal pathogen Fusarium graminearum in crops, providing an environmentally friendly method of resisting fungal pathogens.95 The American cockroach defensin PaDefensin is also a good anti-pathogenic substance. Li et al. predicted the primary and secondary structures and physicochemical properties of PaDefensin and found it to have antiviral effects in both Drosophila Kc cells and embryos.96 Defensins have also been used in tissue engineering. Eslaminezhad et al. prepared a polyethylene oxide (PEO) nanocomposite with wound healing and antibacterial properties using electrospinning technology, and added a composite of copper nanoparticles (CuNPs) and defensin to the material. They found that at a mass of loaded CuNPs/defensin of 1.5/0.5 mg, the composite exhibited antibacterial activity against both Gram-negative and Gram-positive bacteria and potential wound-healing-promoting effects.97 Sanapalli et al. developed a collagen/chitosan composite scaffold coated with poly(lactic-co-glycolic acid) nanoparticles containing HBD2. The scaffold inhibited the survival of Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa, and accelerated the healing of diabetic wounds; the antibacterial, anti-inflammatory, angiogenic, and cell proliferation- and migration-promoting functions of HBD2 were thought to play a role.98
Defensins were initially identified for their antibacterial and antiviral functions. However, with broadening research, the role of defensins in resisting pathogens has been further studied. Moreover, by artificially altering the structure of natural defensins and forming derivatives, their ability to resist pathogens can be improved. Many diseases such as diabetes can cause peripheral nerve disease when they develop to a certain stage. Defensins can impact this progression, and their role in the development and treatment of peripheral nerve conditions resulting from such diseases should also be the target of future research. In this direction, further research is needed on defensin mechanisms of pathogen resistance, in order to clarify the potential of protective mechanisms such as biofilm disruption or pore formation against peripheral nerve diseases. Such research would also enhance our understanding of the functional mechanisms of defensins in mediating the impact of pathogens on peripheral nerve diseases (Figure 5).
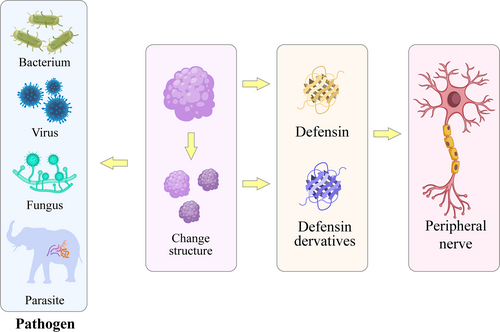
Future research direction: Enhancing the anti-pathogen effects of defensins by altering their structure to target peripheral nerve diseases. Defensins or their derivatives can also affect the progression of peripheral nerve diseases through their resistance to bacteria, viruses, fungi, and parasites.
4 THE EFFECTS OF DIFFERENT DEFENSINS ON PERIPHERAL NERVE DISEASES
The peripheral nerves are mainly composed of myelinated nerve fibers that protrude from neuronal cell bodies and are composed of axons, myelin sheaths, and Schwann cell sheaths. Wallerian degeneration occurs after peripheral nerve injury, leading to pathological changes occurring in all innervated areas. Defensins are active in multiple stages and sites of peripheral nerve fiber lesions, and participate in the pathological and physiological processes of subsequent peripheral nerve disease.16, 99
4.1 The role of α-defensin in peripheral nerve diseases
The Rabbit α-defensin subtype NP1 can strongly influence peripheral nerve injury. A single intramuscular injection of 10 μg/mL NP1 was shown to promote the repair of sciatic nerve clamp injury in rats. Compared to a negative control group at 6 weeks after injury, there was a significant decrease in the absolute value of the sciatic nerve function index, an increase in the thickness of myelin sheaths in the tibial and common peroneal nerves, an increase in the conduction speed of the tibial nerve, and an increase in the wet weight of the tibialis anterior muscle. This process may be related to changes in neural nutrition, inflammation, cell chemotaxis, and expression of cell generation-related proteins.9 When a chitosan cannula was used to repair a 5-mm sciatic nerve defect after sciatic nerve dissociation in rats, the effect of NP1 on the injured sciatic nerve was similar to that of nerve growth factor application at the site of injury. The functional index, number of myelinated nerve fibers, fiber thickness, fiber diameter, myelin sheath diameter, and nerve conduction velocity of the injured sciatic nerve were all restored, and NP1 promoted the regeneration of the sciatic nerve after transection.100 Nozdrachev et al. reported that NP1 increased the growth rate of regenerated nerve fibers by 30% on the 21st day after suturing of the transected sciatic nerve, increased the distance of neural spine conduction, and increased the excitatory conductivity of nerve fibers by 20%, confirming that α-defensin can play a positive role in the recovery of damaged neural stem function.101 Andrianov et al. demonstrated that NP1 reduces the pulse frequency of incoming nerve fibers in a concentration-dependent manner within a concentration range of 0.0001–1 nM. NP1 inhibits the excitatory effect of the neurotransmitter L-glutamic acid and was found to modulate transmission through L-glutamic acid signaling and hair receptors.102 Meanwhile, α-defensin also plays an important role in peripheral nerve-related cells. In the RSC96 Schwann cell line, treatment with 4 μg/ml and 8 μg/ml NP1 for 36 h promoted cell proliferation and migration through the NF-κB signaling pathway, slowing down cell aging and apoptosis.103
These studies demonstrate that α-defensin plays an important role in trauma-induced peripheral nerve disease; the NP1 subtype appears particularly important, acting on the afferent and efferent nerve fibers and effectors in the nerve conduction pathway. Its effect is not inferior to conventional nerve growth factors, which affect the morphology and function of effector muscles by protecting myelinated nerve fibers. We believe that, under the influence of α-defensin, Schwann cells may regulate various related proteins in nerve tissue after peripheral nerve injury. This may include effects on neurotrophic factors, inflammation, cell chemotaxis, and cell generation, through signaling pathways such as NF-κB; in this way, α-defensin ultimately affects Schwann cell proliferation, migration, aging, and apoptosis. Simultaneously, the induced proteins and electrical stimulation can act reciprocally on other locations of the nerve conduction loop and influence neuronal function, forming a virtuous cycle and ultimately promoting the repair of the diseased peripheral nerves. However, there are limited studies on the effects of other α-defensin subtypes on peripheral nerve repair. The different subtypes of α-defensins have the same origin and may also regulate the progression of trauma-induced peripheral nerve disease, representing another important future research direction (Figure 6).
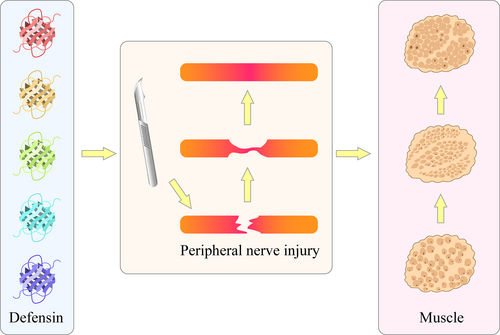
Different types of defensins stimulate the function of regulatory effectors and promote nerve repair after peripheral nerve injury. α-defensin subtypes other than NP1 may also promote peripheral nerve injury repair and the functional recovery of innervated effector muscles.
4.2 The role of β-defensin in peripheral nerve diseases
Rodríguez et al. isolated and purified the paralytic toxin PhcrTx2, and sequence analysis revealed it to be a β-defensin folding peptide with a sequence similar to type 3 potassium channels. PhcrTx2 was shown to inhibit glutamate-gated currents in snail neurons but had little effect on voltage-sensitive sodium or potassium channels or various cloned voltage-gated ion channels in snail and rat dorsal root ganglion cells.104 Tseng et al. investigated the mechanism of itching, which is related to the response of sensory neurons to stimulation. DEFB103 in β-defensins is involved in activating Mas-related G protein-coupled receptors, and plays a role in the activation of mast cells and sensory neurons; specific β-defensins that activate sensory neurons are allergens that cause endogenous itching.105 Patients with Parkinson's disease (PD) have varying degrees of damage to the central and peripheral nervous systems. Researchers have found that levels of β-defensin 2, connexin, and lactoferrin are higher in the intestines of PD patients, indicating a role of β-defensin in PD.106 Cao et al. treated neuroblastoma cells with human β-defensin 4 (HBD4) and found that it inhibited the proliferation of neuroblastoma cells and promoted cell apoptosis by upregulating the Caspase3 protein, which increased significantly with the concentration of HBD4.107 Han Qin et al. confirmed that mouse β-defensin 4 (mBD-4) was inherently expressed in the embryonic nervous system of mice, with its expression increasing with the maturation of the nervous system. After LPS induction, the expression level of mBD-4 in mouse neural tissues was significantly upregulated.108 In addition, some researchers suggest that the antimicrobial peptides human LL-37 and β-defensin 3 can regulate the expression of nerve elongation factor in human epidermal keratinocytes, which indirectly suggests an impact of β-defensin on peripheral nerve-related diseases.109
Therefore, β-defensins also play an important role in peripheral nerve diseases. They may serve not only as a diagnostic marker for such disorders (Figure 7) but also have therapeutic potential. However, in contrast to α-defensins, there is limited research on the role of β-defensins in the repair of trauma-induced peripheral nerve injury; their effects on incomplete and complete peripheral nerve rupture have rarely been reported, and require further investigation (Figure 6).
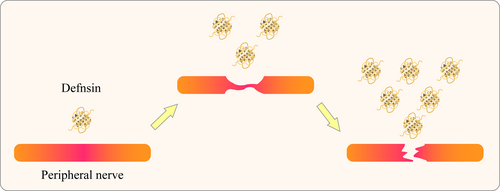
Different types of defensins as diagnostic biomarkers for peripheral nerve diseases. In peripheral nerve injuries of different severity, expression levels of different defensin subtypes may vary, indicating their potential use as detection markers for the progression of injury.
4.3 The role of other defensins in peripheral nerve diseases
Kim et al. isolated a peptide structurally similar to defensins from earthworms and synthesized it into an 11-mer antimicrobial peptide named Lumbricin. This peptide inhibits the proliferation of human neuroblastoma SH-SY5Y cells and reduces apoptosis and viability loss induced by 6-hydroxydopamine, a drug used to model PD. It exerts its neuroprotective effects by the ubiquitination of the p27 (Kip1) protein.[110] In a mouse model of the neurodegenerative disease amyotrophic lateral sclerosis, Wu et al. found increased intestinal permeability, downregulated tight junction protein ZO-1 and adhesive junction protein E-cadherin, increased numbers of Paneth cells, and decreased levels of the antimicrobial peptide defensin 5α. These changes were accompanied by a decrease in quantities of Butyrivibrio fibrisolvens, Escherichia coli, and Firmicutes bacteria.111 Zhu et al. successfully converted insect defensins into neurotoxins. Their team removed the small ring of parasitic wasp venom defensins, which share characteristics with scorpion toxin, thereby eliminating spatial hindrance in peptide-channel interactions. This modification led to neurotoxins that selectively inhibit potassium channels with high affinity, providing evidence of an evolutionary relationship between the two distantly related protein families.112 Meanwhile, Thursted et al. filed for a patent on the blocking effect of θ-defensin-related inflammatory proteases. The patent describes a combination of θ-defensin-related drugs that inhibit tumor necrosis factor-α (TNF α) converting enzyme, pro-inflammatory proteases, and abscisic enzymes. These drugs are intended for the treatment of Alzheimer's disease, inflammation-related neuropathies, and other conditions.113
In summary, evidence from θ-defensins, insect defensins, and plant defensins further suggests a role for these peptides in the occurrence and development of peripheral nerve diseases. Research highlights their ability to regulate cancerous cells, diseased nerves, and effector tissues through interactions with ion channels on the cell surface and gene-regulating proteins in tissues and organs. This evidence suggests that defensins can serve not only as a focus for peripheral nerve disease research but also provide significant translational potential including therapeutic targets and patent opportunities. Clinical applications may include drug development, biomaterial preparations, and diagnostic reagents. Various approaches are being investigated for the future use of defensins in the diagnosis and treatment of peripheral nerve diseases (Figure 8).

Different applications of defensins can be adapted for peripheral nerve disease treatment through the development of medications, scaffold preparations, and diagnostic reagents. Defensins can also have a place in patents or product development related to the treatment of peripheral nerve diseases.
5 CONCLUSIONS AND PERSPECTIVES
The pathogenesis of peripheral nerve diseases is unclear, leading to poor clinical outcomes. Several types of defensins, including mammalian defensins (α-defensin, β-defensin, θ-defensin), insect defensins, and plant defensins, can affect various stages of nerve conduction by regulating inflammatory processes, immune response, and defense against pathogens, thereby affecting the course of peripheral nerve diseases. Cross-species applications, new subtypes of defensins, the construction of defensin derivatives, and the combination of tissue engineering technology with defensins all offer new research directions for the treatment of peripheral nerve diseases. Therefore, progress in research on defensins can advance the clinical treatment of peripheral nerve diseases, benefiting this significant patient population.
AUTHOR CONTRIBUTIONS
Tiantian Qi: Conceptualization; validation; writing—original draft. Qi Yang: Conceptualization; resources; validation; writing—original draft. Haotian Qin: Data curation; resources. Yuanchao Zhu: Data curation; resources. Jin yuan Chen: Data curation; resources. Hongfa Zhou: Data curation; resources. Jian Weng: Conceptualization; resources. Hui Zeng: Conceptualization; funding acquisition; methodology; supervision; validation; visualization; writing—review and editing. Fei Yu: Conceptualization; data curation; funding acquisition; investigation; methodology; project administration; resources; supervision; validation; visualization; writing—review and editing.
ACKNOWLEDGMENTS
This research was supported by grants from Guangdong Basic and Applied Basic Research Foundation (2022A1515220111; 2022B1515120046), National Natural Science Foundation of China (82102568; 82172432), the Scientific Research Foundation of PEKING UNIVERSITY SHENZHEN HOSPITAL (KYQD2021099), Shenzhen Key Medical Discipline Construction Fund (SZXK023), Shenzhen Science and Technology Program (ZDSYS20220606100602005; JCYJ20220818102815033; KCXFZ20201221173411031; JCYJ20210324110214040), and Shenzhen “San-Ming” Project of Medicine (SZSM202211038). We sincerely acknowledge the use of Figdraw (www.figdraw.com) for figure illustration, which greatly facilitated the visualization of our concepts.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
Ethics approval was not needed in this study.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




