Prospects of antidiabetic drugs in the treatment of neurodegenerative disease
Lidan Hu and Wenmin Wang contributed equally to this work.
Abstract
Neurodegenerative diseases (NDs) stand for a group of disorders characterized by the progressive loss of neurons in the brain and peripheral organs, resulting in motor and cognitive dysfunction. The global prevalence of NDs, including Alzheimer's disease, Parkinson's disease, Huntington's disease, and amyotrophic lateral sclerosis, is on the rise globally, primarily due to an aging population, positioning NDs as an increasing significant public health concern. Despite intensive research, few effective therapies that prevent or delay ND progression have been developed. Mounting evidence indicates that one of the well-defined risk factors for NDs is type 2 diabetes mellitus, and insulin resistance has also been proven to be related to cognitive decline. Certain antidiabetic drugs, such as glucagon-like peptide-1 receptor agonists, peroxisome proliferator-activated receptor gamma agonists, and metformin, have shown promise in offering neuroprotective benefits and alleviating ND symptoms beyond their glucose-lowering effects. Although the exact mechanisms remain elusive, these drugs offer a promising novel strategy for managing cognitive disorders. In this review, we first highlight the benefits and specific neuroprotective effects of multiple antidiabetic drugs and discuss the main mechanisms of action of antidiabetic drugs in treating NDs. These mechanisms include reducing protein aggregation and improving apoptosis, mitochondrial dysfunction, oxidative stress, and neuroinflammation. Finally, we summarize clinical trials evaluating these drugs for treating NDs.
Key points
What is already known about this topic?
-
Neurodegenerative diseases (NDs), including Alzheimer's disease, Parkinson's disease, Huntington's disease, and amyotrophic lateral sclerosis, are characterized by progressive neuron loss, which results in motor and cognitive dysfunction. The connection between NDs and type 2 diabetes mellitus has been established, with insulin resistance linked to cognitive decline. Antidiabetic drugs, such as glucagon-like peptide-1 receptor agonists, peroxisome proliferator-activated receptor gamma agonists, and metformin, have shown potential neuroprotective effects, suggesting a possible treatment avenue for NDs beyond their primary role in managing diabetes.
What does this study add?
-
This review highlights the specific neuroprotective effects of various antidiabetic drugs and delves into their mechanisms of action in the context of treating NDs. It offers a detailed analysis of how these drugs can alleviate NDs by reducing protein aggregation, improving apoptosis, and mitigating mitochondrial dysfunction, oxidative stress, and neuroinflammation. Moreover, the review summarizes clinical trials evaluating antidiabetic drugs for treating NDs, offering insights into their efficacy and safety profiles. This comprehensive examination bridges the knowledge gap and could make ways for the development of more effective treatments for NDs leveraging antidiabetic drugs.
1 INTRODUCTION
Neurodegenerative diseases (NDs) are characterized by the deterioration of brain and spinal cord neurons. An individual presents mild symptoms at onset that progressively worsen due to neuronal death.1, 2 Among the degenerative changes in various organs, the nervous system has the most extensive influence on individuals, leading to abnormal changes in motor and cognitive functions. Most NDs are ultimately fatal.3-5 The prevalence of neurodegenerative disorders is expected to dramatically rise worldwide as life expectancy increases. NDs mainly include Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease (HD), and amyotrophic lateral sclerosis (ALS).
The most common neurodegenerative illness affecting the elderly is AD. It is marked by progressive cognitive decline, the aggregation of insoluble amyloid (Aβ), and hyperphosphorylated tau protein accumulation in cortical extracellular plaques and intracellular neurofibrillary tangles.6, 7 It is reported that 50 million people worldwide are affected by dementia, and the number will be about threefold to 152 million by the year 2050.8 PD is the second most prevalent neurodegenerative disorder, which can be distinguished by the progressive degeneration of dopaminergic neurons in the substantia nigra and accumulation of α-synuclein in the form of pathological inclusions.9, 10 HD is an autosomal dominant neurodegenerative disorder with traits of involuntary choreiform movements, cognitive decline, and dementia.11 ALS is a progressive and fatal neurodegenerative disease that leads to paralysis through selective degeneration of motor neurons.12 These diseases substantially burden the healthcare system and economy in our aging society. There is substantial evidence that the aggregation of insoluble Aβ peptides, hyperphosphorylated tau and α-synuclein, mitochondrial dysfunction, apoptosis, oxidative stress, and neuroinflammation contributes to the pathogenesis of most NDs.7, 9, 13-15 Despite concerted research endeavors, effective therapies to halt or slow ND progression remain scarce.
Therefore, it is imperative to investigate and assure the risk factors for NDs to promote the development of therapeutic strategies for these diseases. Beyond well-known factors, such as genetic, aging, environmental, and lifestyle factors, type 2 diabetes mellitus (T2DM) has drawn increasing interest as a potential contributor to ND progression.16 A series of epidemiological studies have shown that T2DM, a metabolic disease characterized by deficient insulin secretion due to impaired insulin sensitivity,17, 18 is a well-defined risk factor for NDs, including AD and PD.19-21 Several epidemiological studies have proved that T2DM increases the risk of progressive AD by 50%–100%.22, 23 People who have diabetes mellitus have a 2–3 times higher risk of having dementia compared to those who do not, and cognitive decline progresses more rapidly among elderly diabetic individuals.23 Moreover, up to 80% of patients with AD, especially older adults over 65, have T2DM or glucose intolerance.24 In fact, AD was proposed to be “type 3 diabetes” in 2005 due to its pathophysiological resemblance to T2DM (reduced insulin production and insulin resistance in AD brains). Diabetes has been deemed as a key risk factor for cognitive impairment, be it mild cognitive impairment (MCI) or dementia. Both conditions are characterized by deficits in verbal and visual memory, attention and concentration, processing speed, executive function, and motor control.25-29 For diabetes and cognitive dysfunction, the association between them is multifaced, involving many mechanisms, such as insulin resistance, chronic inflammation, oxidative stress, protein aggregation, and vascular dysfunction. These shared mechanisms may contribute to cognitive dysfunction and disease progression in both diabetes and NDs, such as AD. Several studies on animal models30-32 and human subjects33-35 have demonstrated that antidiabetic drugs effectively ameliorate ND symptoms. Although the exact mechanisms are not fully comprehended, it is believed that antidiabetic drugs may offer more than neuroprotective effects but also lower blood glucose levels beyond their ability.
This review aims to provide a comprehensive overview of the research progress and mechanisms underlying the use of key antidiabetic drugs to treat NDs. We emphasize the neuroprotective mechanisms of commonly used antidiabetic drugs in NDs, including glucagon-like peptide-1 (GLP-1) receptor agonists, peroxisome proliferator-activated receptor γ (PPARγ) agonists, metformin, bromocriptine, sulfonylureas, and sodium-glucose cotransporter-2 (SGLT2) inhibitors. Our review begins by highlighting the unique benefits and specific neuroprotective effects of these diverse antidiabetic drugs. Subsequently, we provide a comprehensive summary of the mechanisms in terms of how these drugs exert their therapeutic effects on NDs. Finally, we categorize and analyze clinical trials evaluating these drugs. Collectively, antidiabetic drugs present a promising avenue for treating NDs, particularly AD, HD, PD, and ALS.
2 GLP-1 RECEPTOR AGONISTS IN NDS
Glucagon-like peptide-1 (GLP-1) is an endogenous multifunctional peptide secreted by enteroendocrine cells after food intake and is recognized based on its biological effects on regulating insulin secretion, blood glucose, food intake, and body weight.36-38 To date, most GLP-1 analogs, that is, GLP-1 receptor (GLP-1R) agonists, including liraglutide, semaglutide, lixisenatide, albiglutide, and dulaglutide, are approved for the clinical treatment of T2DM.33 GLP-1R is mainly expressed in the intestine and α and β cells of pancreatic islets and it can be found in multiple central nervous system (CNS) regions, such as the hippocampus, cortex, hypothalamus, and key AD- and PD-associated areas.39 In addition, DPP-4 inhibitors increase endogenous incretin levels and are FDA-approved for treating T2DM. These drugs are also potential alternative treatment strategies for NDs.40
GLP-1R agonists have gone through tests in vivo and in vitro in different cell lines, mouse models, and ND patients and have been shown to exhibit neuroprotective properties.41-44 Exenatide treatment halts cognitive decline in Aβ-induced rat and mouse models of AD.45, 46 Similarly, liraglutide, a GLP-1 analog with a longer half-life (∼13 h), ameliorates learning and memory impairment in various models, including the Aβ-induced APP (swe)/PS1 (ΔE9) (APP/PS1) mouse model and the streptozotocin (STZ)-induced AD rat model. Liraglutide also attenuates mutant huntingtin-induced neurotoxicity in human SK-N-MC neuronal cells.47-51 Importantly, the substantial lipophilicity of liraglutide facilitates its peripheral administration and subsequent crossing of the blood‒brain barrier (BBB).52 In the context of PD, exenatide improves dopaminergic neuron degeneration and motor function in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and hydroxydopamine mouse models and a lipopolysaccharide (LPS) rat model.42-44, 53 Liraglutide has further been demonstrated to restore insulin sensitivity and increase cell viability in HD models probably by upregulating antioxidant pathways and reducing oxidative stress. However, the role of GLP-1 receptor agonists in ALS remains less explored. Preliminary data suggest that liraglutide does not significantly alter lumbar spinal motor neurons or glial activation in ALS mouse models.54 Notably, exenatide has also shown potential therapeutic effects in studies of Friedreich ataxia, an autosomal recessive neurodegenerative disease characterized by reduced frataxin protein.[ 55] Exenatide promotes frataxin expression and improves mitochondrial function in patient-derived induced pluripotent stem cell-derived neurons, frataxin-deficient mice, and Friedreich ataxia patients.55
In addition to GLP-1R agonists, multiagonists targeting GLP-1R and glucose-dependent insulinotropic peptide receptor (GIPR) or glucagon receptor (GcgR) are also being explored for treating NDs. Evidence shows that dual GLP-1R/GIPR agonists are more effective than a single GLP-1R agonist in improving motor impairment and inflammation in AD and PD animal models.31, 56 GLP-1R/GcgR preclinical studies in AD and PD cell and animal models have demonstrated that GLP-1R/GIPR/GcgR tritagonists show outstanding neuroprotective effects than a single GLP-1R agonist;57-59 however, the above effects have not been confirmed in humans.
The aggregation of insoluble Aβ peptides, hyperphosphorylated tau, and α-synuclein are critical pathological events in AD and PD animal models.7, 9 GLP-1R agonists ameliorate insoluble Aβ and α-synuclein aggregation and reverse tau hyperphosphorylation via various mechanisms. First, GLP-1R agonists restore impaired PI3K/Akt signaling in the brain and subsequently inhibit the activity of glycogen synthase kinase-3 beta (GSK-3β) by increasing GSK-3β phosphorylation (Figure 1).60, 61 GSK-3β appears to be a crucial kinase for tau phosphorylation and Aβ aggregation, thus reducing AD-related pathology.62 Inhibition of GSK-3β also attenuates rotenone-induced α-synuclein aggregation by promoting autophagy and abrogating α-synuclein-mediated neurotoxicity in PD.63, 64 Second, PI3K/Akt activation upregulates the expression of the insulin-degrading enzyme (IDE) (Figure 1). IDE, the main Aβ-degrading enzyme in the brain, binds and degrades Aβ, subsequently halting the neurotoxic effects of insoluble Aβ.65, 66 Moreover, IDE can inhibit α-synuclein fibril formation in vitro.67 Third, GLP-1R agonists suppress the induction of Jun N-terminal kinase (JNK), preventing Aβ accumulation and tau hyperphosphorylation.68, 69 JNK phosphorylates amyloid precursor protein (APP) and tau protein, promoting Aβ deposition and tau dissociation (Figure 1).70-72 Fourth, exenatide enhances the membrane trafficking and activity of A disintegrin and metalloproteinase domain-containing protein 10 (ADAM10), the α-secretase of APP, by increasing cAMP response element-binding protein (CREB) phosphorylation and brain-derived neurotrophic factor (BDNF) upregulation, which may inhibit Aβ production.73

Mechanism of the neuroprotective action of GLP-1R agonists in NDs.
There is substantial evidence that mitochondrial dysfunction is involved in AD and PD pathogenesis.13-15 GLP-1R agonists have been demonstrated to influence mitochondrial function, including enhancing mitochondrial biogenesis, inhibiting the mitochondrial apoptotic pathway, and reducing oxidative stress across a range of ND models.74, 75 GLP-1R agonist administration enhances the activity of peroxisome proliferator-activated receptor-gamma coactivator 1α (PGC-1α), a key effector of mitochondrial biogenesis.76, 77 PGC-1α activation shields dopaminergic neurons from rotenone- or α-synuclein-induced degeneration.78 The upstream regulation of PGC-1α activity is partially dependent on the deacetylase-Sirtuin1 (SIRT1), a potential therapeutic target for AD79 modulated by GLP-1R agonists in neurons.80, 81 GLP-1R activation reduces the proapoptotic proteins' expression, such as caspase-3, and upregulates antiapoptotic proteins, including Bcl-2 and Bcl-xl, via the PI3K-Akt pathway, thus inhibiting the mitochondrial apoptotic pathway (Figure 1).82-85 In addition, the upregulation of intracellular cAMP induced by GLP-1R elevates the expression of Bcl-2 and Bcl-xl by activating CREB.86 The increase in intracellular cAMP stimulated by liraglutide also activates the mitogen-associated protein kinase (MAPK) pathway, which modulates various intracellular events, such as protein synthesis and inflammation, thus improving neuronal survival.73 Mechanistically, GLP-1R agonists restore multiple processes disrupted in NDs, including protein aggregation, apoptosis, mitochondrial biogenesis, oxidative stress, and neuroinflammation.74, 75
3 PPARγ AGONISTS AND NDS
Peroxisome proliferator-activated receptors (PPARs) are ligand-activated transcription factors that participate in the pathogenesis of a wide range of diseases, such as diabetes, cancer, and NDs.87 Among the three PPAR isoforms, PPARγ is abundant in adipose and brain tissue, especially in neurons, astrocytes, and microglia, and is considered a hopeful target for treating some NDs.88 PPARγ ligands include endogenous or natural agonists and synthetic agonists.89 Synthetic PPARγ agonists are currently successfully used for controlling glucose levels and managing T2DM in the clinic. Synthetic agonists as part of the thiazolidinedione (TZD) family, including pioglitazone, rosiglitazone, and troglitazone, were the first ligand family used as competitive ligands to bind and activate PPARγ.90 Pioglitazone and rosiglitazone exert neuroprotective effects in a series of animal models of PD and AD, significantly improving behavior and motor responses.91-94 TZD and rosiglitazone improve motor deterioration and mutant Htt aggregation in the R6/2 and N171-82Q mouse models of HD.95-98 Pioglitazone delays the onset of ALS and significantly enhances the survival time of superoxide dismutase (SOD1)-G93A transgenic ALS mouse model.99, 100 In addition, pioglitazone exhibits neuroprotective effects and mitigates locomotor dysfunction in TDP-43 and FUS Drosophila models of ALS.101 Numerous studies have substantiated the capacity of these drugs to traverse the BBB. For example, pioglitazone has demonstrated antitumor properties in a glioma xenograft model.102 Nevertheless, the extent of its BBB penetration remains controversial. To resolve this issue, a PPARγ agonist exhibiting superior brain permeability, ELB00824, was formulated; this drug exhibits substantial efficacy in a chronic trigeminal nerve injury model.103
PPARγ agonists promote the formation of a PPARγ heterodimeric complex with nuclear receptor-9-cisretinoic acid (RXR) that binds to PGC-1α and subsequently regulates the transcription of genes related to mitochondrial biogenesis and oxidative stress.88 PGC-1α promotes the expression of downstream target genes involved in mitochondrial biogenesis, such as mitochondrial transcription factor A (TFAM), nuclear respiratory factor 1 (NRF-1), and NRF-2 (Figure 2).104 Moreover, PGC-1α modulates mitochondrial fusion–fission events, thus preventing the reduction of mitochondrial size.105 Activated PGC-1α protects neuronal cells from ROS-induced death through the enhancement of antioxidant enzymes' expression levels, including glutathione peroxidase-1 (GPx1), SOD1, and SOD2 (Figure 2).106
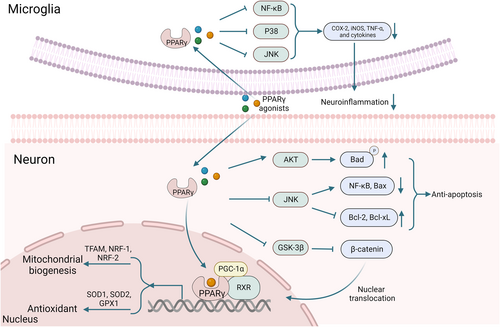
Main potential molecular mechanisms involved in the neuroprotective effects of PPARγ agonists.
Accumulating evidence suggests that neuroinflammation cascade processes are a hallmark of most NDs. Neuroinflammation in NDs is characterized by the activation of astrocytes and microglia.4 Activated glial cells produce proinflammatory factors, such as cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS), and tumor necrosis factor-alpha (TNF-α). These factors contribute to neuroinflammation-induced neurodegeneration. Studies concerning PPARγ agonists have shown to counteract these effects by inhibiting glial cell activation and blocking the production of inflammatory cytokines through the downregulation of p38, nuclear factor-κB (NF-κB), and JNK signaling (Figure 2).107-111 In addition, PPARγ agonists protect neurons from apoptotic cell death by inhibiting JNK phosphorylation, downregulating NF-kB and Bcl-2 associated protein x (Bax) expression, and enhancing B-cell leukemia/lymphoma-2 (Bcl-2)/B-Cell lymphoma-extral-large (Bcl-xL) expression. These agonists also maintain the phosphorylation of the Bcl-2-associated death promoter (Bad) by preserving p-Akt (Figure 2).112-114 In AD, pioglitazone and rosiglitazone have been found to protect hippocampal neurons in Aβ-induced mouse models. These drugs inhibit GSK-3β, facilitating Wnt signaling pathway upregulation and β-catenin translocation from the cytoplasm to the nucleus (Figure 2).115-117 Studies have found evidence on ALS transgenic mice regarding the beneficial effects of PPARγ agonists, partly caused by their anti-inflammatory effects and regulation of the Wnt/beta-catenin pathway. This finding indicates that PPARγ agonists have the potential to be a therapeutic avenue for ALS treatment.118 The interplay between PPARγ and Wnt/beta-catenin signaling has implications for ALS in which PPARγ is downregulated. Understanding this interaction could help in the exploration of novel ALS therapies.89 Activated Wnt signaling exerts neuroprotective effects via Wnt-3a and Wnt-5a. Wnt-3a significantly increases neuron survival by diminishing the activation of caspase-3,119 and Wnt-5a inhibits the synaptic damage and loss induced by Aβ by preventing the decrease in postsynaptic scaffold protein (PSD-95) in hippocampal neurons.120 In HD, the PPARγ agonist rosiglitazone has been found to improve cardiac and muscle function, suggesting its potential role in mitigating systemic symptoms often observed in HD patients.121 PPAR-δ, another isoform, interacts with the mutant huntingtin protein, and its pharmacologic activation has been proved to improve motor function and reduce neurodegeneration in HD mouse models.122 Mechanistically, PPARγ agonists promote the degradation of splicing and transcription aggregate proteins, reduce the neurotoxicity caused by protein aggregation, enhance mitochondrial function, reduce oxidative stress damage to neurons, and contribute to better neuroprotection.
4 METFORMIN AND NDS
Metformin is widely endorsed as the first-line drug for T2DM therapy and exerts its primary hypoglycemic effect by improving insulin resistance and inhibiting hepatic gluconeogenesis.123 Metformin also shows multiple beneficial effects in NDs, such as reducing toxic protein aggregates, promoting neurogenesis, and enhancing neuronal bioenergetics.124-126 In addition, motor function, learning behavior, and memory have been shown to be significantly improved in high-fat diet-fed mice after metformin treatment.81, 127, 128 Metformin protects dopaminergic neurons from death,129-131 and long-term metformin treatment remarkably enhances locomotor and muscular activities in the MPTP mouse model of PD.131 Interestingly, the survival time of male but not female transgenic HD mice has been found to be noticeably prolonged by long-term metformin feeding,132 indicating that sex potentially influences metformin function. Notably, metformin does not reduce pathology in the female SOD1G93A mouse model of ALS, even inhibiting estrogen production.133
For a long time, metformin has been recorded to exert various neurophysiological effects by permeating the BBB and activating specific neurons and glial cells,134 a claim supported by clinical trials demonstrating cognitive improvement.135 To date, metformin's mechanism of action in NDs remains elusive. One of the most widely studied mechanisms of action is AMP-activated protein kinase (AMPK) activation.126, 136 Metformin activates the AMPK signaling pathway by inhibiting mitochondrial respiratory chain complex I and increasing the AMP/ATP ratio.136 Here, we highlight some potential mechanisms of action of metformin according to the literature. Similar to GLP-1R agonists, metformin plays a role in regulating Aβ and α-synuclein aggregation and tau phosphorylation.126, 137 Mechanistically, metformin ameliorates Aβ deposition in AD mouse models, which may be mediated by the strengthened expression of IDE or decreased expression and activity of β-secretase 1 (BACE1), a key enzyme in the cleavage of APP required to generate Aβ (Figure 3).138-140 Moreover, metformin has been demonstrated from further studies to increase the dephosphorylation of α-synuclein and tau significantly by activating protein phosphatase 2A (PP2A) in the brain (Figure 3).141, 142 Metformin has also been seen to reduce neuronal loss, rescuing cognitive deficits in AD by dampening Aβ-induced apoptosis. Metformin protects against Aβ-induced apoptosis by inhibiting the JNK/mitogen-activated protein kinase (MAPK) signaling pathway143 and reducing the activity of caspase3/9 in an AMPK-dependent manner (Figure 3).144 Furthermore, growing evidence indicates the beneficial anti-neuroinflammatory effects of metformin; neuroinflammation is recognized as an initiating trigger in AD and PD, and the effects of metformin on this process cannot be neglected.145 Metformin significantly reduces the expression of proinflammatory cytokines, including TNF-α, interleukin-1 beta (IL-1β), and iNOS, in the MPTP model of PD,146 potentially regulated by the AMPK/NF-κB signaling pathway (Figure 3).140 Notably, a new finding demonstrated that metformin enhances the secretion of GLP-1 by connecting insulin and Wnt signaling, potentially exerting a regulatory effect on GLP-1R and subsequently resulting in neuroprotective effects.147 Overall, metformin lowers blood glucose levels, enhances the function of neuronal mitochondria, inhibits neuronal death, suppresses Aβ formation, and reduces neuroglial inflammatory responses.
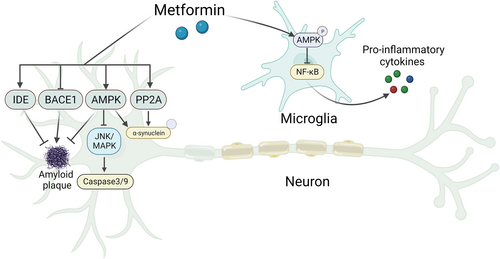
A schematic model of the mechanism of metformin in neurons and microglia.
5 BROMOCRIPTINE AND NDS
Bromocriptine is an ergot derivative dopamine receptor agonist approved by the FDA for treating T2DM.148, 149 Bromocriptine improves hyperglycemia, glucose tolerance, and insulin sensitivity in people with T2DM.150 Bromocriptine administration augments low hypothalamic dopamine levels and suppresses hepatic glucose production, reducing postprandial plasma glucose levels.149, 151 The accumulation of dopamine in pancreatic β-cells decreases insulin secretion in mice following an injection of L-3,4-dihydroxyphenylalanine (L-DOPA) alone,152, 153 suggesting that although insulin secretion is regulated by glucose to a large extent, it can also be improved via the dopaminergic system.154 Furthermore, dopamine mediates insulin release in autocrine and paracrine manners. Similarly, bromocriptine acts as a dopamine receptor 1 (D1R) antagonist in beta cells and a D2R agonist in delta cells, attenuating glucose-stimulated insulin secretion from beta cells.155
While bromocriptine is known to demonstrate a limited capacity to penetrate the BBB, lately, studies have suggested that it can cross the BBB when used as a substrate in endothelial cells with high expression of organic anion-transporting polypeptides (OATPs) 1A2 and 2B1.156 Advances in nanomaterial development have also facilitated optimized drug delivery across the BBB.157 The neuroprotective effect of BRO is not less than partially due to the reduction in glutamate-induced neurotoxicity and upregulation of the antiapoptotic protein Bcl-2 resulting from activation of the PI3K cascade in a D2 dopaminergic receptor-dependent manner (Figure 4).158 In addition, bromocriptine upregulates the expression and activity of the antioxidant enzyme NAD(P)H quinone oxidoreductase 1 (NQO1) by enhancing the expression and nuclear translocation of nuclear factor-E2-related factor-2 (Nrf2), which is mediated by the PI3K/Akt signaling pathway and independent of D2 receptor activation, thus protecting neuronal cells against oxidative stress (Figure 4).159 Moreover, bromocriptine treatment suppresses glial cell inflammation and oxidative damage by decreasing the quantity of reactive astrocytes, downregulating the expression of inflammatory factors, such as iNOS and TNF-α, and upregulating the levels of antioxidant proteins, including transcription factor 3 (ATF3) and heme oxygenase-1 (HO-1) (Figure 4).160 Bromocriptine, a cholinergic drug, offers numerous benefits to patients with T2DM, such as lowering postprandial blood glucose levels by promoting low levels of hypothalamic dopamine and inhibiting liver glucose production, thereby improving hyperglycemia, glucose tolerance, and insulin sensitivity.148-151 Bromocriptine also acts as an agonist of specific dopamine receptors, demonstrating sedative effects and promoting neuronal regeneration.161 Moreover, bromocriptine reduces glutamate-induced neurotoxicity and upregulates the antiapoptotic protein Bcl-2, thus demonstrating neuroprotective properties.158
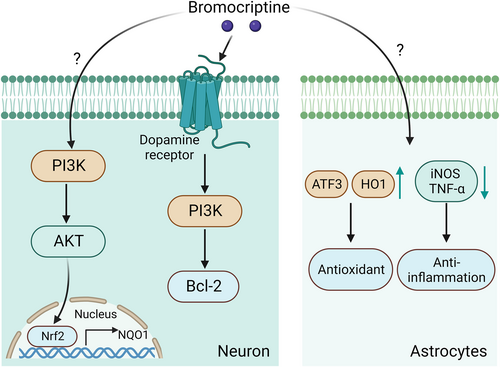
Potential signaling pathway in neurons and astrocytes treated with bromocriptine.
6 SULFONYLUREAS AND NDS
Sulfonylureas are a class of oral hypoglycemic agents commonly used to manage T2DM. Their primary mode of action involves stimulating pancreatic β-cells to close ATP-sensitive K-channels (KATP) in the cytoplasmic membrane. This membrane depolarization subsequently opens calcium channels, resulting in a growth in calcium influx and intracellular calcium, triggering insulin release and lowering blood glucose levels. Recently, the hidden effect of sulfonylureas in the cure of NDs has attracted attention. For instance, a Mendelian randomization study revealed an association between genetic variants targeting sulfonylureas and a reduced risk of attention deficit disorder.162 Another study suggested that sulfonylureas improved AD outcomes, although the exact mechanisms remain unclear.163 Some evidence suggests that sulfonylureas may protect nerve cells by mitigating oxidative stress and inflammatory responses. A study on T2DM patients who developed primary intracerebral hemorrhage (pICH) revealed reduced ICH and peripheral brain edema (PHE) volumes, potentially through the modulation of MMP-9, an inflammatory modulator.164 Moreover, sulfonylureas may also improve mitochondrial function in NDs.165, 166
Sulfonylureas have also shown neuroprotective effects. While these drugs are not traditionally expected to have access to cross the BBB, recent studies have begun to explore their potential in neuroprotection.167 For example, glibenclamide has demonstrated neuroprotective effects in animal models of ischemic stroke, although its ability to cross the BBB remains a subject of ongoing investigation.168 Further studies are needed to elucidate the mechanisms that are used by sulfonylureas to affect neural pathways. In the context of AD, sulfonylureas may inhibit the production and aggregation of beta-amyloid and the hyperphosphorylation of tau proteins. KATP channels have been implicated in Aβ-induced pathology.169 Studies have shown that sulfonylureas, such as glimepiride and glibenclamide, can protect against Aβ-induced synaptic damage and restore BBB Aβ transport, respectively.170, 171 However, research on the cognitive effects of sulfonylureas in T2D patients remains limited.172 In PD, sulfonylureas may inhibit mitochondrial dysfunction and reduce oxidative stress.173, 174 These drugs may also enhance the survival of dopamine neurons.175 For example, K-ATP channels in midbrain dopamine neurons are sensitive to sulfonylureas and may protect against hypoxia or hypoglycemia (Figure 5).176 Regarding HD, sulfonylurea gliclazide increases the firing rate and regularity of STN neurons in 5–7-month-old BACHD mice, an effect independent of Epac2/Rap1 signaling. This finding suggests a potential therapeutic role for gliclazide in HD via a KATP channel-independent pathway. A meta-analysis also demonstrated that the regular use of medications such as sulfonylureas may prevent ALS (Figure 5).177 Despite the promising potential of sulfonylureas in treating NDs, current research is preliminary. Further clinical trials and laboratory studies are needed to validate the efficacy as well as the safety of these drugs for NDs.

The mechanism of the neuroprotective function of sulfonylureas in NDs.
7 SGLT-2 INHIBITORS IN NDS
SGLT-2 inhibitors, compared to others, are a relatively new class of antidiabetic drugs primarily used for treating T2DM. These drugs function by inhibiting glucose reabsorption in the renal tubules, lowering blood glucose levels. Recently, the potential neuroprotective effects of SGLT-2 inhibitors in NDs have garnered research interest.178 In the context of AD, SGLT-2 inhibitors could offer multiple benefits through various mechanisms.179 These drugs demonstrate anti-inflammatory, antioxidant, and atheroprotective effects and direct neuroprotective activities, such as increasing BDNF levels and inhibiting acetylcholinesterase (AChE).178 Notably, insulin resistance is a common issue in AD, affecting approximately 8 in 10 patients. This resistance extends to the central nervous system, leading to reduced glucose metabolism in AD patient brains. Insulin resistance is connected with the activation of GSK3-β signaling, a pathway implicated in tau phosphorylation, Aβ production, and Aβ-mediated neuronal damage (Figure 6).180 Studies have shown that SGLT-2 inhibitors can reduce GSK3β activity in hepatocytes. In a mouse model study, treatment with an SGLT-2 inhibitor significantly reduced the pathological changes associated with attention deficit disorder, including tau phosphorylation and age spot density.181 These changes were correlated with improvements in cognitive functions, such as the ability to remember and learn, as assessed by the novel object recognition and Morris water maze tests.182 Although originally designed to block glucose reabsorption in the kidneys, some SGLT-2 inhibitors, such as empagliflozin, have shown promise in preclinical studies for NDs.183 Despite their generally low lipophilicity, which makes it challenging for them to cross the BBB, they may still offer neuroprotection through indirect mechanisms such as anti-inflammatory effects. Current studies are actively exploring ways to modify the molecular structure of these drugs, potentially enhancing their ability to penetrate the BBB.
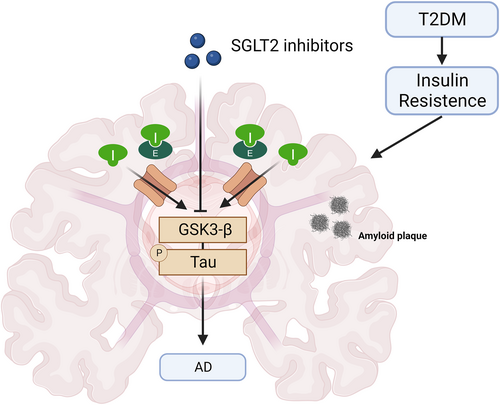
The mechanisms by which SGLT2 inhibitors improve cognition across the blood‒brain barrier in NDs.
In conclusion, the emerging role of SGLT-2 inhibitors as a novel class of antidiabetic agents with potential applications in NDs is attracting considerable attention. Preliminary findings suggest that these drugs may offer neuroprotective effects through multiple mechanisms, although further research is needed for the confirmation of these effects and a fully understand their implications.
8 CLINICAL TRIALS EVALUATING ANTIDIABETIC DRUGS FOR TREATING NDS
Several clinical studies have indicated that antidiabetic drugs may minimize the risk of cognitive impairment in people with T2DM. We have summarized existing clinical trials evaluating antidiabetic drugs for treating NDs in Table 1, including completed and ongoing studies.
| NDs | Drug Name | Patent Name | Patent Number | Title of clinical trial | Clinical Trial/Phase | Status |
|---|---|---|---|---|---|---|
| AD | Liraglutide | Treatment of NDs | WO/2017/213,524 | Evaluating liraglutide in AD | NCT01843075/2 | Ongoing |
| Semaglutide | GLP-1R agonist and methods of treatment | US20210353710A1 | A research study investigating semaglutide in people with early AD (EVOKE plus) | NCT04777409/3 | Recruiting | |
| Semaglutide | Pharmaceutical composition comprising sustained-release microspheres including GLP-1 analogue or pharmaceutically acceptable salt thereof | US20230096928A1 | A research study investigating semaglutide in people with early AD (EVOKE) | NCT04777396/3 | Recruiting | |
| Exenatide | Combination | WO/2021/005,147 | A pilot clinical trial of Exendin-4 in AD | NCT01255163/2 | Terminated | |
| Pioglitazone | Methods of treating neurological, metabolic, and other disorders using enantiopure deuterium-enriched pioglitazone | US20160331737A1 | Pioglitazone in AD | NCT00982202/2 | Completed | |
| Pioglitazone | Methods and drug products for treating AD | WO/2013/106,084 | Pioglitazone in early PD | NCT01280123/2 | Completed | |
| Rosiglitazone | Methods of treating or preventing AD using indane acetic acid derivatives | US20140086910A1 | Rosiglitazone as monotherapy in subjects with mild to moderate AD | NCT00428090/3 | Completed | |
| Rosiglitazone | Compositions for the treatment of neurodegenerative conditions and methods for the use thereof | WO/2009/126,444 | Rosiglitazone as adjunctive therapy in subjects with mild to moderate AD | NCT00348140/3 | Completed | |
| Rosiglitazone | Study of rosiglitazone XR in subjects with mild-to-moderate AD | NCT00550420/3 | Terminated | |||
| Rosiglitazone | Open-label extension study of rosiglitazone XR As Adjunctive therapy in subjects with mild-to-moderate AD | NCT00490568/3 | Terminated | |||
| Metformin | Compositions and methods for the treatment of NDs | US20220305006A1 | Effect of insulin sensitizer metformin on AD biomarkers | NCT01965756/2 | Completed | |
| Empagliflozin (SGLT2i) | Sodium-glucose Co-transporter 2 (sGLT2) inhibitor and endogenous ketone production | NCT03852901 | Completed | |||
| PD | Lixisenatide | A method for reversing aging brain functional decline | WO/2022/127,868 | Study to evaluate the effect of lixisenatide in patient with PD | NCT03439943/2 | Ongoing |
| Semaglutide | Semaglutide depot systems and use thereof | WO/2023/281,495 | GLP1R in PD | NCT03659682/2 | Not yet recruiting | |
| Exenatide | Drug cocktail for treatment of PD, lewy body disease and multiple system atrophy | WO/2022/224,118 | Exenatide once weekly over 2 Years as a potential disease modifying treatment for PD | NCT04232969/3 | Active, not recruiting | |
| Exenatide | Combination therapy for NDs | WO/2023/042,178 | Exenatide treatment in PD | NCT04305002/2 | Active, not recruiting | |
| Exenatide | Methods for treating diabetes with extended release formulations of GLP-1 receptor agonists | WO/2012/177,929 | Trial of exenatide for PD | NCT01971242/2 | Completed | |
| Exenatide | Long-acting GLP-1R agonist as a therapy of neurological and neurodegenerative conditions | US11123405B2 | Exendin-4 as a treatment for PD | NCT01174810/2 | Ongoing | |
| Exenatide | Effects of exenatide on motor function and the brain | NCT03456687/1 | Completed | |||
| Bromocriptine | Liquid pharmaceutical compositions | US20220117959A1 | Ophthalmologic safety of long term treatment with pramipexole compared to bromocriptine or other dopamine agonists in patients with PD | NCT02233023/4 | Completed | |
| Bromocriptine | Bromocriptine compsns. For oral admin - with controlled release properties, useful for treating Parkinson's disease, hyper-prolactinaemia etc. | CH669113A5 | Pramipexole and bromocriptine on nonmotor symptoms of early PD | NCT01673724/4 | Completed | |
| Bromocriptine | New compositions for treating NDs | WO/2012/117,073 | Safety and efficacy of pramipexole and bromocriptine combined With L-dopa in PD | NCT02172573/3 | Completed | |
| Bromocriptine | Randomized single-blind placebo controlled comparative trial of pramipexole and bromocriptine in PD | NCT00240409/3 | Completed | |||
| Sulfonylurea | Correlation between idiopathic Parkinson's disease and diabetes mellitus | Completed | ||||
| ALS | Pioglitazone | PPAR agonist compositions and methods of use | WO/2010/073,235 | Study of pioglitazone in patients with ALS | NCT00690118/2 | Terminated |
| HD | Metformin | Neuroprotection by mitochondria-targeted metformin | US20190091245A1 | Testing metformin against cognitive decline in HD | NCT04826692/3 | Recruiting |
Considering the protective effects and potential mechanisms of GLP-1R agonists in distinct ND models, as described above, several clinical trials have taken place to evaluate GLP-1R agonists for treating PD and AD, demonstrating significant effects.35, 184 Exenatide significantly improves PD patients' motor and cognitive function, according to a single-blinded clinical trial in 45 patients with moderate PD (NCT01174810).35 A double-blind phase II clinical trial in over 200 AD patients showed that liraglutide protected against cognitive impairment (NCT01843075).184 Other clinical trials of GLP-1R agonists in PD and AD are currently underway, including two phase III clinical trials evaluating the drug semaglutide for treating AD patients (NCT04777396 and NCT04777409) and a series of clinical trials evaluating GLP-1R agonists for treating PD patients (NCT03659682, NCT04305002, and NCT04232969).
Clinical trials evaluating different PPARγ agonists for treating different NDs have yielded inconsistent results.185-188 In a recent retrospective investigation, the incidence rate (IR) of PD in the glitazone treatment group was reduced by 28% compared with that in those prescribed other antidiabetic treatments (IRR 0.72).185 A prospective cohort study which involved 145,928 patients demonstrated that the cumulative long-term use of pioglitazone reduced the dementia risk by 47% (RR = 0.53, p = 0.029) relative to that in nondiabetics.186 However, pioglitazone did not affect progression in patients with early PD.187 Pioglitazone combined with riluzole also had no positive benefit on the survival time of ALS patients.188
Most clinical studies have shown that metformin can be a safe and well-tolerated medication in older individuals with diabetes. However, clinical trials assessing the therapeutic effects of metformin on NDs have yielded conflicting results. Metformin reduced the high-risk incidence of PD in a study of T2DM patients in Taiwan,189 exerting a cognitive benefit.190 However, metformin has an insignificant effect on T2DM patients with AD or amnestic mild cognitive impairment (aMCI),191 and long-term metformin treatment for T2DM is associated with a slightly higher risk of the development of AD.192 A study published in the Journal of Alzheimer's Disease revealed that using metformin is associated with a reduced risk of developing dementia in people with T2DM.193 Therefore, the mechanism of action of metformin on NDs requires further study, which might help explain the contradictory clinical effects of metformin for treating these disorders.
9 DISCUSSION
NDs, including AD, PD, HD, and ALS, cause gradual structural and functional deterioration of neurons, resulting in functional impairment.1, 2 These diseases have become a significant burden on modern healthcare systems and the economy, with no effective cures available to prevent or delay their progression. Diabetes is a global health concern that affects a vast population, with over 80% of patients with AD also demonstrating T2DM or impaired fasting glucose.194 Moreover, diabetes is an important risk factor for several neurological disorders, including PD, HD, ALS, and ischemic stroke.19, 21 Due to the correlation between NDs and diabetes, researchers have aimed at the potential application of antidiabetic drugs to treat NDs. This review summarized the research progress and mechanisms of action of antidiabetic drugs in the treatment of NDs, focusing on the mechanisms of GLP-1R agonists, PPARγ agonists, metformin, bromocriptine, sulfonylureas and SGLT-2 inhibitors.
Clinical trials have shown significant effects of GLP-1R agonists in PD and AD, and several new clinical trials evaluating the treatment of these conditions with GLP-1R agonists are currently underway.35, 184 Similarly, synthetic agonists belonging to the TZD family and pioglitazone have shown neuroprotective effects in many PD and AD animal models.91-98 These findings suggest that these antidiabetic drugs may provide new treatment options for patients with NDs. Regarding NDs such as AD, PD, HD, and ALS, antidiabetic drugs such as GLP-1R agonists and PPARγ modulators have shown promise due to their ability to target common pathological pathways, including inflammation and oxidative stress, offering a unified therapeutic strategy. Several interconnected mechanisms underlie the potential effectiveness of antidiabetic drugs in treating NDs. First, insulin resistance, a hallmark of T2DM, is increasingly associated with cognitive dysfunction and AD development. Insulin plays multiple roles in the brain, including neurotransmitter release, synaptic plasticity, and memory formation.195 Second, diabetes and NDs are characterized by chronic inflammatory responses. Hyperglycemia can activate inflammatory cells, leading to neuroinflammation, which plays the role of a potential link between these two conditions.196, 197 Third, increased oxidative stress is generally observed in diabetes patients and may be linked to oxidative damage in the brain, contributing to the progression of NDs.198 Fourth, abnormal protein aggregation is a shared feature of diabetes and NDs, such as AD. For example, the aggregation of β-amyloid in AD is akin to the anomalous folding and aggregation of insulin in diabetes.199 Finally, diabetes can lead to microvascular complications that may affect cerebral blood supply and neural function.200
In recent years, considerable attention has been directed toward the gut–brain and lung–brain axes in the study of NDs. For instance, research has revealed relations between gut microbiota imbalances and various central nervous system disorders, including PD; however, studies in humans remain preliminary.201 Studies have also underscored the pivotal role of the gut–brain axis in NDs, such as AD and PD, and in T2DM. GLP-1-based antidiabetic drugs and metformin hold promise for positively influencing the gut–brain axis, opening new avenues for ND treatment. Specifically, these drugs regulate blood glucose and body weight and improve gut flora. This mechanism can alleviate the metabolic dysfunction triggered by antipsychotic medications. For example, clinical trials have demonstrated that exenatide improves patient motor and cognitive functions over time.75 This improvement may be attributed to the drug's role in modulating the gut–brain axis, which influences metabolism. Several additional double-blind clinical trials of GLP-1R agonists, including exenatide, for the treatment of PD and other NDs are either underway or soon to be initiated.202 Antipsychotic drug therapy can alter the metabolism and composition of the gut microbiota. Metformin, for instance, not only partially reverses alterations in the gut microbiota caused by treatment with second-generation antipsychotics (SGAs) but also actively corrects disturbances in peripheral and central satiety-related neuropeptides. The antidiabetic effects of metformin are associated with the activation of AMP kinase in the hypothalamus and impact gut flora.203 Thus, the gut–brain axis has emerged as a promising research area for treating NDs.204 We have emphasized the neuroprotective mechanisms of commonly used antidiabetic drugs in NDs in Table 2. However, other antidiabetic drugs for treating NDs are still in the preliminary stages of mechanistic research and may achieve their therapeutic effects through a combination of mechanisms, including modulation of these two axes. As previously discussed, these antidiabetic drugs can modulate tau hyperphosphorylation and other degenerative processes, further emphasizing their potential interaction with the BBB. While the exact mechanisms and extent of BBB interaction with these antidiabetic drugs remain under investigation, their demonstrated efficacy in ND models offers a promising avenue for future research. Subsequent studies should aim to elucidate these mechanisms, paving the way for novel therapeutic strategies to treat NDs.
| NDs | Major antidiabetic drug | Experimental models | Therapeutic Potentials | Refs |
|---|---|---|---|---|
| AD | Exenatide | MPTP mouse | Halts cognitive decline | [41] |
| LPS rat | ||||
| Human | ||||
| Liraglutide | MPTP mouse | Ameliorates the learning and memory impairment | [42-45] | |
| STZ-rat model | ||||
| SK-N-MC neuronal cells | ||||
| Rosiglitazone | Mice | Promote autophagy and reduce proinflammatory markers | [99-102, 104] | |
| Pioglitazone | APPswe/PS1∆e9 mice | Protect the hippocampal neurons | [127-129] | |
| Metformin | Mouse | Ameliorates the deposition of Aβ, increased the dephosphorylation of α-synuclein and tau | [147-149] | |
| Sulfonylurea | Pharmacological | Inhibit the neurotransmitter degrading enzyme acetylcholinesterase (AChE) | [205] | |
| SGLT2i | Scopolamine-induced rat | Improve performance and lower risk of dementia | [206; 207] | |
| Human | ||||
| PD | Exenatide | MPTP mouse | Improve dopaminergic neurons degeneration and motor function | [39] |
| LPS rat hydroxydopamine mouse | ||||
| Liraglutide | MPTP mouse | Protects and refines the motor function | [47] | |
| SK-N-MC neuronal cells | ||||
| Rosiglitazone | Rat (6-OHDA) | Inhibited 6-OHDA-induced activation of microglia in the striatum | [47, 99-101, 104] | |
| Pioglitazone | Mice | Motor phenotypes were improved by reduction of neuroinflammation | [47, 99-101, 104] | |
| Metformin | MPTP mouse | Protect dopaminergic neurons from cell death | [140-142, 145] | |
| Bromocriptine | Human | Suppresses the inflammation of glial cells and the oxidative damage | [160] | |
| Sulfonylurea | Human | Enhance the affinity of the subunit of the KATP-channel | [208] | |
| SGLT2i | PD mice | Improve mitochondrial function and antioxidative effect | [209] | |
| Human | ||||
| HD | Rosiglitazone | Mouse | Mproved motor deterioration and mutant Htt aggregation | [105-108, 209] |
| Metformin | Mouse | Gender dependency patterns | [143] | |
| Bromocriptine | Human | Reduction of glutamate-induced neurotoxicity and the upregulation of antiapoptotic protein Bcl-2 | [158] | |
| Sulfonylurea | Human | Ameliorate neurodegenerative disorders | [210] | |
| SGLT2i | HD rat | Reduce inflammation, autophagy and promote behavioral outcome | [211] | |
| ALS | Pioglitazone | Mouse | Neuroprotective effects and mitigates locomotor dysfunction | [76, 77, 212] |
| Metformin | SOD1G93A mouse | No beneficial effects | [95] | |
| Bromocriptine | Mouse human | Protect motor neurons from the oxidative injury via suppression of astrogliosis | [160] |
We acknowledge several limitations associated with this review. First, some reports and clinical trials claim that antidiabetic drugs have no effect on ND treatment and even exhibit contradictory results, potentially due to different sample sizes, differences in the course and heterogeneity of pathogenesis, and difficulty in controlling other potential interfering factors in clinical trials. Second, this review lacks relevant evidence on the impact of other types of diabetes medications on NDs. Therefore, addressing these limitations and thoroughly assessing the advantages and disadvantages of antidiabetic drugs for clinical application requires further systematic research. Additionally, vigilance for potential side effects is crucial when contemplating the use of antidiabetic drugs for NDs, especially in nondiabetic patients. Notably, the dosages and treatment regimens required for NDs may differ from those used for diabetes, potentially resulting in a distinct side effect profile. Furthermore, the chronic nature of NDs necessitates long-term treatment, raising concerns about extended drug use. Continuous monitoring and regular follow-ups are imperative to ensure patient safety.
10 CONCLUSION
In conclusion, this review offers new ideas and strategies for treating NDs. Antidiabetic drugs may offer new therapeutic approaches for patients with NDs. Through continuous in-depth research, effective treatment measures can be developed to alleviate the serious harm caused by NDs, providing patients with better medical services and a higher quality of life. In detail, this review mainly focuses on the research progress and mechanisms of action of antidiabetic drugs in treating NDs. T2DM is associated with growth in the risk and clinical severity of NDs. Moreover, antidiabetic drugs, including GLP-1R agonists, PPARγ agonists, metformin, and bromocriptine, exhibit neuroprotective roles in cell and mouse models of NDs and ND patients. The underlying mechanisms by which antidiabetic drugs regulate neuroprotection mainly involve reducing protein aggregation and improving apoptosis, mitochondrial dysfunction, oxidative stress, and neuroinflammation. Further exploration of the mechanism of action of antidiabetic drugs is required, focusing on how they act on neurons and glial cells and identifying their targets with neuroprotective effects. Experimental models provide valuable insights that are crucial for addressing this challenge. However, it is essential to enhance collaboration between experimental researchers and clinical practitioners to ensure the effective translation of these insights into specialized treatment and prevention methods.
AUTHOR CONTRIBUTIONS
Lidan Hu: Conceptualization; Project administration; Writing – original draft. Wenmin Wang: Writing – original draft. Xiangjun Chen: Writing – original draft. Guannan Bai: Writing – original draft. Liangjian Ma: Investigation. Xin Yang: Investigation. Qiang Shu: Conceptualization; Writing – review & editing. Xuekun Li: Conceptualization; Writing – review & editing.
ACKNOWLEDGMENTS
We thank the National Clinical Research Center for Child Health for their support. This study was financially supported by the National Key Research and Development Program of China (grant number 2017YFE0196600 to X.L.), the National Natural Science Foundation of China (grant number 92049108 to X.L.), the National Natural Science Foundation of China (grant number 82000030 to X.Y.) and the Natural Science Foundation of Zhejiang Province of China (grant number LQ22C070004 to L.H.).
CONFLICT OF INTEREST STATEMENT
The authors report no conflicts of interest.
ETHICS STATEMENT
Ethics approval was not needed in this study.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




