Do all creatures possess an acquired immune system of some sort?
Abstract
Recent findings have provided evidence for the existence of non-vertebrate acquired immunity. We survey these findings and propose that all living organisms must express both innate and acquired immunity. This is opposed to the paradigm that only vertebrates manifest the two forms of immune mechanism; other species are thought to use innate immunity alone. We suggest new definitions of innate and acquired immunity, based on whether immune recognition molecules are encoded in the inherited genome or are generated through somatic processes. We reason that both forms of immunity are similarly ancient, and have co-evolved in response to lifestyle, cost-benefit tradeoffs and symbiosis versus parasitism. However, different species have evolved different immune solutions that are not necessarily genetically related, but serve a similar general function – allowing individuals to learn from their own immune experience; survival of species is contingent on the acquired immune experience of its individuals.
Abbreviations
-
- BCR
-
- B-cell receptor
-
- CRISPR
-
- clustered regularly interspaced short palindromic repeats
-
- DAMP
-
- danger-associated molecular pattern
-
- DSCAM
-
- Down syndrome cell adhesion molecule
-
- FREP
-
- fibrinogen-related protein
-
- NK
-
- natural killer
-
- NKT
-
- natural killer T
-
- PAMP
-
- pathogen-associated molecular pattern
-
- PRR
-
- pattern recognition receptor
-
- RAG
-
- recombination activating genes
-
- R genes
-
- resistance genes
-
- RM
-
- restriction modification
-
- RNAi
-
- RNA interference
-
- TCR
-
- T-cell receptor
-
- VLR
-
- variable lymphocyte receptor
Introduction
The immune system has several roles: protection against parasitism by viruses, bacteria, and foreign or aberrant cells; repair of organ and tissue damage; and maintenance of integrity and regeneration 1. Different types of living organisms – from unicellular bacteria and archaea to multi-cellular plants and animals – have different immune needs. In general, however, the current paradigm, which is based primarily on the vertebrate immune system, divides immunity into two arms or sub-systems: innate immunity and acquired immunity. What we here shall call “acquired immunity” is usually termed “adaptive immunity”; but we believe the adjective “acquired” is more suitable than “adaptive”, since, from an evolutionary perspective, even innate immunity is adaptive.
It has been assumed that the innate sub-system is evolutionarily more ancient, and that variants of this system function in plants, invertebrates, and vertebrates 2. In contrast to innate immunity, acquired immunity has been seen as an evolutionary innovation that is restricted to vertebrates. However, similar to vertebrates, invertebrates, and plants are also exposed to continuous assault from various parasites, and they too must heal wounds and manage symbiotic relationships in a multi-organismal ecosystem; even prokaryote bacteria and archaea have to deal with parasitic viruses 3. Is it possible to maintain life in the face of these external threats and internal needs without having some kind of acquired immune system? We review recent findings that provide evidence for the existence of non-vertebrate somatically acquired immunity, and discuss why we believe that some form of acquired immunity is essential.
We propose that all species must express the two forms of immune mechanisms. Different acquired mechanisms complement innate mechanisms by allowing individuals to learn from their own immunological experience on a time scale that is much shorter than the evolution of the species. Acquired immunity, as we define it below, faces challenges new to the evolutionary experience of the species by employing various somatic mechanisms that offer specificity and diversity, far beyond those offered by non-immune allelic variation. Acquired immunity ensures that a sufficient number of individuals can survive the challenge, allowing the species to adapt evolutionarily on longer time scales. Survival of the species, in other words, is contingent on the immune experience of its individual members, and individual immune experience can only be gained by somatically generated immune mechanisms.
An alternative view of the immune system
We begin with a primary definition that differentiates between innate and acquired immunity, based on whether the immune recognition molecules are encoded or not encoded directly in the inherited genome (Fig. 1). This definition is independent of the specific molecular and cellular mechanisms by which this recognition is realized. We sharpen the details as we proceed.
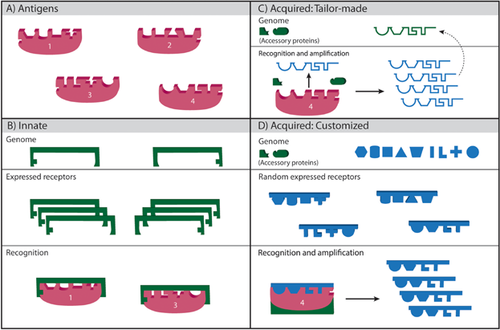
Innate immunity uses immune molecules that are encoded in the inherited genome as fully functional molecules. Innate immune recognition molecules recognize classes of potential pathogens and internal signals of injury or aberration in need of immune attention; thus, innate immunity targets characteristic “biomarker” molecules. Innate immune mechanisms evolve on the time scale of species.
Acquired immunity uses immune recognition molecules that are not pre-encoded in the inherited genome as complete molecules, but are somatically created through various innate mechanisms deployed by individual members of the species. These mechanisms generate somatic immune recognition molecules by exploiting genomically inherited subunits, or by using pathogen-derived molecules as templates. Each acquired immune recognition molecule can recognize a specific target molecule. Acquired immunity evolves somatically at the time scale of individuals.
Note that acquired immune processes also employ accessory molecules – effectors and others, which are innately encoded in the inherited genome. The point is that the somatically acquired molecules confer discrete specificity to the immune recognition process. The two systems also appear to differ functionally: innate immune mechanisms are typically poised for immediate deployment and can be effective as a first line of response; however, these ready-made mechanisms are not as specific as are acquired mechanisms. Acquired immunity exhibits memory of previous immune challenges during the lifetime of the individual, which renders acquired immunity more effective upon repeated challenge. Acquired immunity must deploy mechanisms to ensure self-tolerance, although even innate immunity must be regulated to avoid destruction of self. We argue that these functional characteristics are not in themselves defining, but are operational consequences of our fundamental definition.
Non-vertebrate immune mechanisms
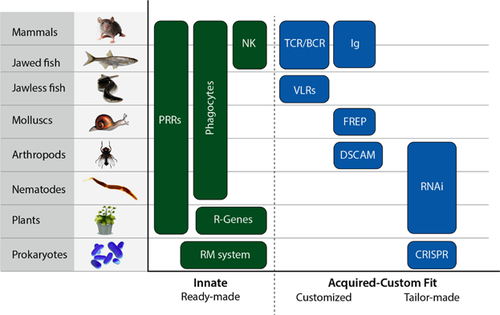
On the basis of the definition given above, we briefly survey some of the latest findings of non-vertebrate somatically acquired immunity, together with some representative innate immune mechanisms. From this phylogenetic evidence, we may conclude that all classes of living organisms express both sub-systems of immunity; we suggest that innate and acquired immunity are similarly ancient (Fig. 2). We also provide some evidence for immune phenomena that are not well understood, but may point the way for additional acquired mechanisms yet to be discovered.
Prokaryotes
Both bacteria and archaea deploy innate defense mechanisms against phages and viruses 3. For example, the restriction modification (RM) system is a broad-spectrum innate immune system that targets and cleaves DNA. Methylation of the prokaryote's own DNA marks it as “self” and prevents it from being targeted for cleavage 3. However, it was recently found that prokaryotes also deploy acquired immunity, which results from the immune experience of the prokaryotic cell: CRISPR (clustered regularly interspaced short palindromic repeats) 4 is a family of several immune mechanisms that has been found in about half of all bacterial species studied, and in almost all archaea 5-8. Short segments of specific foreign DNA, known as spacers, are reversibly incorporated into a dedicated “cassette” in the prokaryotic genome, and function as a heritable immunological memory of past exposures to specific phage and plasmid infections. CRISPR insertions are then used to recognize and silence specific exogenous genetic elements upon secondary challenge. Recognition of exogenous DNA, based on DNA complementarity, leads to destruction of the foreign DNA via the recruitment of a complex of innate host proteins. As expected, prokaryote autoimmunity and cell death resulting from self-targeting CRISPR spacers have been reported 9. Hence, a tight regulation of this system is needed, and indeed, the first description of CRISPR self-non-self discrimination was reported in Staphylococcus epidermidis 10. In bacteria, horizontal gene transfer of CRISPR has also been reported 11, 12.
Plants
Plants deploy innate immune mechanisms 13 such as pattern-recognition receptors (PRRs). PRRs enable the detection of pathogen-associated molecular patterns (PAMPs) and trigger an immune response 14. Plants can discard a damaged part or mount systemic acquired resistance (SAR), which is a long-lasting, broad-spectrum, systemic immune response. Plants also deploy an acquired anti-viral defense system based on tailored RNA interference (RNAi) molecules 15 that provide systematic, sequence-specific acquired immunity. The RNAi somatic sequences are derived from viral parasites using innate mechanisms, and they determine specific recognition of foreign homologous RNA; this acquired recognition, in association with innate effector proteins, results in specific inactivation of viruses 16. We discuss RNAi in more detail in Box 1, and below in relation to arthropods.
In addition, plants have developed intracellular immune receptors known as resistance (R) proteins, which can recognize certain pathogen molecules and trigger effector immunity that is highly polymorphic for different pathogen strains. R genes, which are the most abundant and diverse of plant gene families, have been found to reside in clusters within plant genomes 17. This suggests the existence of some means for diversification of R genes and subsequent selection for greater specificity. However, the mechanisms generating R gene diversity are not well understood. Autoimmune reactions have also been observed in plants, and have been attributed to hybrid necrosis resulting from intra-specific or inter-specific crosses 13; R genes have been found to be involved in this phenomenon. Indeed, the over-expression of an R protein can also result in autoimmunity 13. And finally, tight regulation of R proteins has recently been reported 18. Note that although R genes manifest some functional characteristics of acquired immunity, we currently define R genes as innate, because a mechanism for generating their somatic diversity has not been discovered. Lastly, there is evidence that plants are capable of specific immune responses, and that they often generate long-term “memory” of previously encountered pathogens 13, 19; such memory can be transgenerational 20 through chromatin modifications of the plant genome 21, 22. However, the limited mechanistic knowledge regarding generation of these responses precludes their classification as acquired, according to our strict definition.
Cnidaria
Cnidaria constitute one of the earliest evolutionary branches in the animal kingdom. These animals mount several types of innate immune response, including the use of PRRs 2. Note that all multi-cellular organisms in our survey use PRRs (Fig. 2), and so we shall not discuss these further for other phyla.
Specific acquired immune mechanisms in Cnidaria have yet to be characterized. However, there are some findings that hint at the existence of such mechanisms. It is known that Cnidaria can discriminate between self and non-self in an unknown way 23. There is also evidence for pathogenic autoimmunity in Hydractinia echinata (snail fur) colonies, which misuse their incompatible-colony defense system to actively destroy their own tissues 23. Interestingly, this form of autoimmunity in H. echinata has been identified as maternally inherited and arises coincidentally with the acquisition of reproductive maturity; the timing is reminiscent of known autoimmune diseases more prevalent in human women 24, 25, despite the vastly different biology.
Nematodes
Animals in this phylum produce specialized immune cells. Caenorhabditis elegans has six coelomocytes, which are innate immune scavenger cells that maintain the worm by endocytosing fluid from its body cavity 26. C. elegans also uses acquired RNAi immunity against viruses 27, 28; such tailored viral silencing agents were shown to be inherited across generations 29.
Arthropods
Arthropods have several types of hemocytes that are involved in immunity; for example, their plasmatocytes are analogous to the phagocytes of other taxa 30. In addition to plants and nematodes, acquired RNAi immunity has also been identified in many insects 31, including Drosophila melanogaster 32, 33. RNAi mounts and amplifies a highly specific response against viral pathogens by using small interfering RNA fragments that are derived from viral mRNA as specific templates for identifying those forign mRNAs, leading to their destruction 34 (see also Box 1). RNAi requires careful regulation to avoid energetically costly runaway amplification, and also to guard against self-directed reactions, which, indeed, have been identified 34.
RNAi and mammalian acquired immunity
Different acquired immunity systems seem to have some common functional analogies. As an example, we compare RNAi to mammalian T-cell immunity 34, 82. The RNAi response to a foreign virus is triggered by the recognition of viral dsRNA by host innate receptors of the Dicer family proteins. Upon recognition, dsRNA is sliced by Dicer proteins into small fragments (typically 20–25 base-pairs long) called siRNA, which are highly specific to the original virus. It has been suggested that Dicer proteins act analogously to innate pattern-recognition receptors (PRRs), detecting viral dsRNA as pathogen-associated molecular patterns 82, 83. The siRNA fragments serve as acquired immune recognition molecules, and specificity is provided by base-pair complementarity with the viral gene. siRNA molecules bind members of the Argonaute protein family in an RNA-induced silencing complex (RISC). This complex serves as an innate effector that silences viral targets, with specificity provided by the siRNA 82. siRNA can be amplified by RNA-dependent RNA polymerases, thus boosting the RNAi response to the viral mRNA.
The mammalian immune response is initiated by recognition of either pathogen-associated or danger-associated molecular patterns, by innate PRRs. The mammalian immune system prepares in advance a large variety of randomly made somatic T-cell receptors (TCR), carried by T cells, using the innate RAG1/2 mechanism. Here, the T-cell receptors serve as acquired immune recognition molecules, and specificity is provided by the sequence and structure unique to each receptor. Upon recognition of an antigen, with the assistance of the innate MHC complex, antigen specific T-cell clones are selected for the immune response. These clones proliferate to amplify an acquired immune response. Useful clones are preserved for future needs as immune memory, similarly to the specific siRNAs in the RNAi system 29.
Moreover, the D. melanogaster immune system features a mechanism to generate acquired immunity based on the DSCAM (Down syndrome cell adhesion molecule) gene 35, which encodes an immunoglobulin super-family (IgSF) protein. In D. melanogaster, DSCAM can undergo alternative splicing, potentially generating tens of thousands of protein isoforms 36; this degree of transcript diversity has not been found in the mammalian homolog of DSCAM. It was later shown that Anopheles gambiae DSCAM produces pathogen-specific splice-form repertoires upon immune challenge 37, which suggests that it functions as an acquired immunity mechanism 38.
As with other phyla, there is evidence that implicates immune memory in arthropods. The bumble bee Bombus terrestris shows specificity in protection upon secondary exposure to bacterial pathogens 39. Similarly, priming D. melanogaster with a sub-lethal dose of Streptococcus pneumoniae protects against an otherwise-lethal second challenge of the pathogen 40. Likewise, evidence for immune memory was recently found in A. gambiae 41. The exact molecular and cellular mechanisms responsible for specificity and memory in these cases are not fully understood.
Molluscs
Molluscs, similarly to arthropods, possess different types of hemocytes involved in immunity, some of which are phagocytic 30. Acquired diversity generated somatically by alternative splicing has been identified in fibrinogen-related proteins (FREPs) in the snail Biomphalaria glabrata 42. FREPs are composed of amino-terminal IgSF domains and carboxy-terminal fibrinogen-related sequences. They are up-regulated upon parasitic invasion, and can bind to parasites or their products. Individual snails can differentially express the alleles of different FREP subfamilies, and a high level of diversity was shown to be generated by somatic point mutations and alternative splicing 43-45.
Tunicates and cephalochordates
In addition to innate immune PRRs and phagocytes, Branchiostoma floridae (amphioxus) also has an immune-type gene family that encodes variable region-containing chitin-binding proteins (VCBPs) 44. A large number of VCBP alleles and haplotypes have levels of polymorphism exceeding of the number found in the “standard” amphioxus genome 46. VCBPs have been found to be secreted into the gut lumen of Ciona; these molecules bind bacteria in vivo, and have been shown to promote bacterial recognition and phagocytosis by granular amoebocytes in vitro 47. We currently define VCBPs as innate, since a mechanism for generating somatic diversity has not been discovered.
Vertebrate jawless fish
These fish use a molecular mechanism of acquired immunity similar to the RAG1- and RAG2-mediated VDJ rearrangement characteristic of mammals, but their rearranging receptors are encoded by a different gene family known as variable lymphocyte receptors (VLRs) 48, 49. Two lineages of lymphoid cells that resemble the adaptive T and B cells of jawed vertebrates have been identified in sea lamprey (Petromyzon marinus) 50. The finding of the VLR system, alongside the discovery of a RAG-like gene cluster in echinoderms 51, have undermined the “big bang” theory of adaptive immunity 52. This theory proposed that adaptive immunity was launched when the genome of an ancient vertebrate was invaded by a transposon that encoded RAG, followed by whole genome duplications, that paved the way for the production of rearranged VDJ receptors 53.
In summary, almost all of the species discussed – from prokaryotes to vertebrates – feature both innate and acquired immunity. The literature also reports more examples of early evolutionary characteristics of acquired immunity 54-57, and we expect that more evidence and mechanisms will be forthcoming. This may take time: bacteria have been under intensive research for over a century, but CRISPR was appreciated less than a decade ago.
But from what we already know, it seems that innate and acquired immunity have been partners from the beginning of the evolution of life; they have each evolved increasing complexity but have never stopped working together. Furthermore, the observations already in hand clearly challenge our ability to draw a sharp line between innate and acquired immunity featured in various species, as the terms are generally defined today 2. Even the distinctions between innate and adaptive immunity in vertebrates have become blurred 58, 59 by the discovery, for example, of mammalian marginal zone and B1-type B cells 60 or NKT cells 61. Hence, we think that sharper definitions and terminologies are required (see also Box 2).
Custom-fit immunity: Fit to meet the challenge
We believe that recent findings across the plant and animal kingdoms call for new definitions of innate and acquired (adaptive) immunity. A better definition for an unambiguous distinction between mammalian innate and adaptive immunity has been proposed 58. However, we think that the continued use of innate and adaptive terms for all new findings might restrict our thinking, in particular because adaptive immunity has been historically considered to be restricted to vertebrates.
We suggest the following terms to accommodate new thinking. Ready-made immunity can be used to describe generic immune mechanisms that are genomically inherited, encoded and ready for immediate deployment, such as pattern-recognition receptors. Custom-fit immunity, by contrast, describes immune mechanisms that are not pre-coded in the inherited genome, but are generated in response to various somatic experiences. Custom-fit immunity can take either a Customized form, as in the example of mammalian clonal expansion of B and T cells, or it can be fully Tailor-made as in RNAi or CRISPR.
A useful metaphor could be taken from the clothing industry. A new customer can immediately buy and wear ready-made, standard-sized clothes. But he or she will probably be best fitted with tailor-made clothes, which take longer to produce and are much more expensive. Customized clothing is based on pre-prepared patterns, modified to fit each customer. A customized suit can be prepared much faster than a tailor-made suit, but not as fast as when buying ready-made clothing off the rack. It should be noted, however, that once measurements have been taken, making new custom-fit clothes is much faster, provided that the customer maintains the same size and figure.
All organisms manifest acquired immunity that must work in concert with innate immunity
It should be emphasized that acquired immunity alone is not enough; the immune system must include both innately genomic and somatically acquired mechanisms that function in concert. Obviously, acquired immunity always requires some innate mechanisms to initiate and regulate the somatic acquisition or rearrangement processes, as we mentioned above. Execution of immune function, like destruction of a target upon recognition, also may require innate mechanisms that follow recognition mediated by acquired immune molecules. The dependency of acquired immune mechanisms on an innate infrastructure, however, should not lead us into mistakenly classifying processes such as RNAi or DSCAM, for example, as solely innate. But why did acquired immunity develop in the first place?
The main reason, we believe, is simple: no species can survive unless a sufficient number of its member individuals can survive immune challenges that are new to the evolutionary experience of the species. And for this, somatic mutations or acquisitions are needed, as we will explain below. As mentioned above, the immune system has several roles 1. There are ongoing routine tasks, such as maintenance and the regulation of organismal integrity, together with episodic protection from invaders and the need to be prepared for unforeseen – even unforeseeable – troubles. The unforeseen, by definition, can demand huge resources for preparedness and immediate action. The information carried by a genome borne by an individual organism is limited by constraints of space and complexity and so no genome could ever encode a complete innate immune system sufficient – a priori – to deal with every immune contingency. Additionally, a species whose immune system is solely genetically encoded and is limited to genomic mutation and individual selection is likely to need more time to adapt genomically to new challenges than it may take for the species to be eliminated by evolving parasites. Acquired specificity, on the other hand, enables the production of a much greater number of reactivity patterns than does the genome alone (Box 3).
Mechanisms of acquired immunity: Information capacity and genome size
Specific recognition by acquired immune molecules requires the generation of a huge potential diversity. For example, recognition of any 20- to 25-base nucleic acid sequence (as typically provided by RNAi), requires 420–425 (equivalent to 1012–1015) different molecules. Similar sized repertoires can also be generated by the vertebrate V(D)J recombination process 81. The size of any genome is limited and precludes encoding such large repertoires. For example, encoding the entire 20-base sequence repertoire of RNAi would require 20 × 1012 (=2 × 1013) nucleotides, which is much larger than any arthropod or even the human genome. Hence, various mechanisms have evolved to enable the somatic generation of such repertoires. One consequence of these mechanisms is that the repertoire of somatic recognizing molecules expressed by each individual at a given time is only a fraction of the potential repertoire that can be generated by the somatic process (and which is potentially expressed by the species). This contrasts with innate recognizing molecules, which are generally expressed by all individuals within the species; polymorphic innate molecules are expressed only by fractions of the species.
Above all, somatically acquired immunity quickly builds immunity from individual experience, enabling the organism to survive new threats that the species did not encounter previously, such as a newly mutated pathogen. Acquired immunity ensures the survival of some individuals, which buys the time needed by the collective species to adapt to the new threat, and transfer its experience to its offspring; obviously an acquired immune molecule that subsequently enters the inherited genome would fit the ideas of Lamarck and coworkers 62-64. Indeed, CRISPR is an example of a form of acquired immunity that is incorporated into the genome to become inherited. RNAi viral silencing agents are known to be inherited across generations in C. elegans 29. We have mentioned above the transgenerational memory of plants 20, and there is similar evidence in arthropods 56. Vertebrates also use their somatically acquired diversity to influence the germ line genome. For example, the acquired TCR repertoire influences the frequency of innate MHC alleles in specific populations faced with novel epidemic diseases 65, 66. Hence, somatic acquired immunity is used for adaptation of the species.
Effective immunity has also to be economical; investment in immunity must be balanced with other fitness traits. An organism needs to eat, grow, differentiate, reproduce, and so on. Immune mechanisms can also damage the organism by direct or collateral effects 67, and recovery from an immune response should require minimum effort. Again, the solution is to combine the innate with the acquired. Innate mechanisms are poised for more generic and immediate deployment, as a first line of response, and for ongoing maintenance tasks. Acquired immunity is more specific than innate immunity and thus saves resources – by amplifying only required specific agents – and also spares collateral damage. Moreover, acquired immune molecules and cells can be safely removed when the organism returns to routine maintenance, thereby reducing maintenance costs.
Acquired specificity allows the allocation of immune responses to several specific efforts, even contradicting ones, and enables decision-making in parallel 1. This enables an immune system to effectively carry out two (or more) different immune efforts simultaneously, and to fight pathogens while managing a delicate symbiosis with related commensals. Finally, specificity makes possible immune memory, which enhances the effectiveness of a response to a repeated challenge. But specificity itself, although a must, has its costs: uncontrolled somatic rearrangement increases the risk of autoimmunity 34, and uncontrolled mutations in a complex organism increase the risk of cancers 68. Hence, specificity must be accompanied by tight regulatory mechanisms; again, some of them are innately encoded, such as in the MHC.
To sum up, survival of the species is contingent on the immune experience of its individual members, and individual immune experience can only be gained by varied somatic immune mechanisms, working effectively in concert with innate immunity.
Different acquired immune solutions are needed for different species and lifestyles
Our brief summary shows that different species have evolved different acquired immune solutions that are not homologous or genetically related; one did not evolve from the other. Each type of acquired immunity evolved separately, but each serves a similar generic function – allowing individuals to learn from their own immune experience and adapt accordingly, on the time scale of a lifetime.
Growing complexity, both internal and environmental, has marked the advance of immune system evolution: Internally, organisms have evolved compartmentalized functions for food intake, reproduction, proliferation, and differentiation. Environmentally, organisms have evolved in response to an ongoing “arms race” with pathogens 53 that survive by avoiding or sabotaging host immunity 34, 69. But successful organisms also need to manage a delicate symbiosis with commensals – the microbiome demands exquisite immune regulation 70. There is no reason to suspect that creatures less complex than jawed vertebrates were exempt from the pressures exerted by both pathogens 44 and symbionts 71. For example, bacteria and plants harbor viral symbionts 72; bacterial symbionts are essential for nitrogen fixation in plants 73 and for the metabolic needs of animals generally.
Simple organisms house fewer cells and tissues and so have simpler internal maintenance problems. For bacteria, the relatively simple acquired immune solution offered by CRISPR is sufficient; CRISPR targets DNA or RNA threats, and can also be incorporated into the genome and transmitted to future generations. Simple multi-cellular invertebrates began to generate specialist innate immune cells, and they also evolved another basic acquired immune mechanism – RNAi. Both CRISPR and RNAi molecules are templated by specific polynucleotide molecules of the pathogen. The strategy is to await an external cue (such as a pathogen challenge) before engaging in tailor-made mass production of acquired immune molecules that are pathogen specific. In this way, there is no need to regularly maintain idle acquired molecules.
But these solutions use simple RNA strands that are relatively limited in their effector mechanisms and are directed mainly against viruses. Each tissue in a complex organism might require a different immune strategy to deal with different maintenance tasks (skin compared with brain, e.g.) and to control different parasites and symbionts (gut compared with skin, e.g.). Animals such as arthropods and molluscs have developed alternative splicing methods to generate acquired immune molecules (DSCAM and FREPs, respectively); although they are more restricted than vertebrates in their combinatorial diversity, such mechanisms may suffice for their internal complexity and their variety of prokaryote pathogens. Note that arthropods deploy both RNAi against the more diversified viruses, and DSCAM-mediated acquired immunity. We anticipate that further similar combinations are waiting to be discovered.
More complex, multi-cellular organisms such as vertebrates need to deal with micro-organism pathogens and symbionts, and also to maintain many more different tissue types 74. So new somatic methods for generating combinatorial diversity evolved. The strategy of these more complex organisms has been to prepare in advance, using somatically generated mechanisms, a large variety of recognizing molecules (potentially randomly made) at low copy number. Upon demand, the system moves to mass production, and can advance to customized specificity – an example is somatic hyper-mutation and affinity maturation in B cells. Useful patterns are preserved for future needs as immune memory. Adaptive immune cells such as lymphocytes have evolved to join innate cells and express a variety of immune responses, such as T helper 1 (TH1) and TH2 type, or regulatory Tr cell responses.
We do not know whether vertebrates, like simpler organisms, also use RNAi for acquired immunity 75. RNAi has a dual use in species such as arthropods, in both defense and development. However, in vertebrates, to our knowledge, RNAi is much more extensively used for developmental and physiological processes. We reason that RNAi-based immune systems were abandoned by vertebrates because the plethora of vertebrate viruses could have evolved mechanisms to sabotage RNAi-based defense systems 76 and, thus, endanger RNAi-based development and physiology. Lymphocytes would appear to have provided vertebrates with a more effective and less dangerous means of acquired immunity. Emerging specialization is also evident in innate systems: TLR molecules were first discovered in D. melanogaster where they function both in development and in immunity 77; mammals have evolved to use TLR molecules primarily for immunity 14.
More research is required to establish our understanding of the evolution of different immune solutions. However, the evolution of particular immune mechanisms must also have adapted to life-management factors such as life expectancy, time to reproductive age, offspring size, the ability to repair, regenerate, or dispose of damaged organs and so forth. A timely immune response is also needed, and different creatures have varied needs in this respect. Reptiles are a telling example: although they have a vertebrate acquired immune system, they lack lymph nodes and do not form germinal centers 78, which are used in mammals as sites of interactions between innate and acquired immune cells. Being ectothermic, reptiles may use temperature-induced fluctuations as a defense mechanism, and make do with comparatively slower immune responses 78. Hence, they probably do not need these complex mammalian immune “meeting places” to speed up the initiation of the acquired phase of their immune response.
Conclusions
Here we have discussed why we think that all living organisms need to express both innate and acquired immunity. We have proposed a new definition for the terms innate and acquired immunity; rather than defining the terms by any particular molecular mechanism or functional arguments, we base the distinction on whether the defining immune recognition molecules are encoded directly in the inherited genome of the species (innate, ready-made immunity), or are generated by a somatic process at the level of the individual organism (acquired, custom-fit immunity). Thus, the vertebrate adaptive immune system can be viewed as one example of a larger class of custom-fit immune systems. This is not merely a semantic difference, but a conceptual one. We believe that updated definitions better incorporate the latest findings across the plant and animal kingdoms and can help guide us to new research questions. New research is required to establish the mechanistic foundations of the different acquired immune systems 79, which in our mind have been overlooked because, in part, of the inadequacy of current definitions and the prevailing paradigm.
We have also discussed the conditions and tradeoffs that could have shaped immune system evolution in different species. Despite incomplete information, we reason that common principles have shaped the evolution of immune systems in all creatures. Indeed, better understanding of other immune systems will provide new insights into our own immunity. For example, a more comprehensive view of immune adaptation can prompt us to uncover the mechanisms that generate congenital IgM and IgA autoantibody repertoires – developing in utero – that are shared between the cord bloods of unrelated human newborns 80.
Finally, it seems that only one other physiological system has evolved to enable a species to exploit individual experience beyond the information encoded innately in the inherited genome: the central nervous system. The immune system and the nervous system are unique in having evolved to provide the organism and hence the species with information beyond the bare genomic endowment, and it will surely be illuminating to further explore their mutual evolution 1.
Acknowledgments
We thank Robert Fluhr and Rotem Sorek for helpful discussions, and Ishai Sher for graphical assistance. NF is incumbent of the Pauline Recanati Career Development Chair of Immunology.