Tannic acid extracted from gallnut improves intestinal health with regulation of redox homeostasis and gut microbiota of weaned piglets
Abstract
The objective of this study is to evaluate the effects of tannic acid (TA) derived from gallnut supplementation on growth performance and health status of weaned piglets. A total of 432 weanling piglets (7.05 ± 1.05 kg) were randomly allocated into 4 treatment groups with 6 replicates of 18 pigs/pen. Piglets were fed either a basal diet (CON), or basal diets supplemented with 1.5 kg/t TA, 3.0 kg/t TA, or 1.8 kg/t zinc oxide (ZnO) for 21 days. The results showed that, compared to the CON, dietary TA supplementation did not affect (p > 0.05) growth performance and serum biochemistry of weaned piglets. However, 3.0 kg/t TA had higher SOD, GPX, and CAT activities and a lower MDA concentration in the jejunum than those of the CON or the ZnO group. Meanwhile, 3.0 kg/t TA increased (p < 0.05) villus height and villus height/crypt depth, and decreased (p < 0.05) crypt depth in the small intestine. Dietary TA also downregulated (p < 0.05) IL-1β and TNF-α expression in jejunum. Furthermore, 3.0 kg/t TA reduced (p < 0.05) the abundance of Candidatus Brocadia and Escherichia-Shigella in cecal digesta. Notably, both Candidatus Brocadia and Escherichia-Shigella had a negative correlation with antioxidant enzymes activities (R < −0.60, p < 0.01), but Escherichia-Shigella was positively correlated with MDA concentrations (R = 0.44, p < 0.05) in the jejunum. In conclusion, compared to the CON, 3.0 kg/t TA supplementation improved the gut health status of weaned piglets, potentially by regulating redox homeostasis and gut microbiota.
INTRODUCTION
The early weanling offers several benefits in swine production, including improved sow reproductive performance, shortened production cycles, and reduced spread of infectious diseases [1]. However, weanling piglets are susceptible to weanling stress, which can disrupt redox balance, compromise gut barrier function, and impair gut microbiota homeostasis [2-4]. These effects ultimately lead to reduced growth performance, increased incidences of diarrhea, and even mortality in piglets [5]. Notably, antibiotic drugs, widely applied to alleviate weanling stress and promote growth in weaned piglets, have been banned due to concerns about antibiotic-resistant pathogens and antibiotic residues in food [6]. Subsequently, high doses of zinc oxide (ZnO) have been used as an alternative to antibiotics in weaned piglets [7]. However, the use of high doses of ZnO poses risks such as toxic effects in piglets, environmental pollution, and the spread of heavy metal tolerance genes [8, 9]. Therefore, developing safe and effective feed additives to improve intestinal health and growth performance of weaned piglets is of great significance for swine husbandry and food security.
Tannic acid (TA) is a naturally occurring polyphenolic compound mainly extracted from legumes, gallnuts, sorghum, and medicinal herbs [10]. Traditionally, TA has been considered an anti-nutritional factor due to its ability to reduce nutrient digestibility by forming precipitates with proteins and digestive enzymes [11]. However, studies increasingly found that appropriate doses of TA can reduce diarrhea incidence and improve gut health without any adverse effect on the growth performance of piglets [12-15]. The observed positive effects of TA on weaned piglets can be attributed to its polyphenolic hydroxyl structure, which exhibits strong physiological properties such as anti-diarrhea, bacteriostasis, and antioxidant activities [16, 17]. Besides, TA have found to have similar functions as a prebiotic, capable of regulating gut microbiota [18]. Strikingly, the European Food Safety Authority has approved TA as a safe new feed additive [19], further supporting its potential benefits for animals as an alternative to antibiotics.
Gallnut, an important Chinese herbal medicine, has been known to promote the growth and development of livestock by regulating their intestinal health. However, the effects of TA derived from gallnuts on weaned piglets is not well understood. A recent study reported that the appropriate level of dietary TA supplementation would be from 0.1% to 0.2%, which could effectively improve gut health of weaned piglets [12]. Therefore, the objective of this study is to investigate the potential effects of dietary supplementation with 1.5 and 3.0 kg/t TA purified from gallnuts on the growth performance, redox homeostasis, and gut health in weaned piglets.
MATERIALS AND METHODS
Animal trial and sample collection
A total of 432 Pig Improvement Company (PIC) weanling pigs (7.05 ± 1.05 kg) were randomly allotted into 4 dietary treatment groups with 6 replicates of 18 pigs/pen (9 barrows and 9 gilts). The piglets from the four groups were fed a basal diet (Table S1) supplemented with 0 (CON), 1.5, and 3.0 kg/t TA (Wufeng Chicheng biotech Co., Ltd., Yichang, China), or 1.8 kg/t zinc oxide (ZnO) for 21 days. Feed and water were given ad libitum, and feed intake, diarrhea, and body weight of piglets were measured at day 0 and day 21 for the calculation of average daily gain (ADG), average daily feed intake (ADFI), and feed/gain ratio (F/G). On the last day of the trial, 6 piglets per treatment were selected based on the average body weight and slaughtered after 8 h of feed deprivation. Fallowing, the serum, jejunum, and cecum digesta samples were immediately collected and stored at −80°C for subsequent biochemical and gut microbial analysis [20, 21]. The small intestinal parts (duodenum, jejunum, and ileum) were perfused with ice-cold isotonic saline and fixed in 4% paraformaldehyde for subsequent histologic examination.
Serum biochemistry and antioxidant parameter analysis
The activities of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP), along with concentrations of total protein (TP), albumin (ALB), total bilirubin (TBIL) blood urea nitrogen (BUN), creatinine (CREA), and glucose (GLU) in the serum were determined using an automatic biochemistry analyzer (Beckman Synchron CX4 PRO, Fullerton, CA) as previously described [22]. The antioxidant parameters including super oxidase dismutase (SOD), glutathione peroxidase (GPX), catalase (CAT), and malondialdehyde (MDA) in the serum and jejunum digesta were determined using their specific assay kits (A001-3, A005-1, A003-1, and A007-1) as previously described [20]. All specific kits were purchased from the Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Small intestinal histologic analysis
Small intestinal histopathology and morphology were analyzed as previously described [20]. Briefly, the fixed duodenum, jejunum, and ileum tissues were embedded in paraffin, sectioned at 5 μm, and then stained with hematoxylin and eosin. All slides were examined using a microscope, and the villus height (VH) and crypt depth (CD) of each slide were measured using Image J software.
Western blotting analysis
Total proteins of jejunum tissues were extracted for western blotting analysis. The primary antibodies used in the western blot assays were shown as follows: IL-1β antibody (ABclonal, A23484), IL-6 antibody (Proteintech, 21865-1-AP), TNF-α antibody (Proteintech, 17590-1-AP), Occludin antibody (ABclonal, A24601), and Claudin-2 antibody (ABclonal, A14085). All protein expression levels were normalized to that of the housekeeping protein β-actin (ABclonal, AC026), and densitometric quantification of the western blotting bands was determined by Image J software [23].
16S rRNA gene sequencing and microbiome analysis
The cecal microbiota was analyzed as previously described [24]. Briefly, total bacterial DNA in the cecal digesta was extracted and amplified with the primers 338F (5′-ACTCCTACGGGAGGCAGCA-3′) and 806R (5′-GGACTACHVGGGTWVTAAT-3′). The sequenced library was established with the TruSeq Nano DNA LT Library Prep Kit (Illumina, USA), and all amplicons were sequenced using an Illumina MiSeq/NovaSeq platform following the standard protocols. After sequencing, the raw data were demultiplexed using the demux plug-in, and quality-filtered, denoised, and merged using the DADA2 plug-in [25]. The microbiota composition, alpha diversity index (Shannon index and observed OTUs), and beta diversity based on Jaccard distances, and principal coordinate analysis (PCoA) were estimated using the OmicStudio tools [26]. Linear discriminant analysis (LDA) effect Size (LEfSe) analysis with LDA score >2 was employed to identify differences in taxonomic abundance between groups [27]. Correlation analysis was generated by Spearman's rho correlation test, and only correlations with coefficients < −0.4 or >0.4 and p-values <0.05 were considered significant. Significant differences between groups were calculated using the Kruskal–Wallis test, and false discovery rate (FDR) adjusted p values were corrected using the Benjamini–Hochberg method [28].
Statistical analysis
Statistical analyses were performed using SPSS version 19.0 (Chicago, IL, USA). Levene's test of homogeneity of variance was used to evaluate unequal variances before ANOVA analysis. Data were analyzed by one-way ANOVA, followed by Duncan's multiple range test for multiple mean comparisons. Data are presented as mean ± SD, with a significance level of p < 0.05, and tendency was set at 0.05 ≤ p < 0.10.
RESULTS
Growth performance, serum biochemistry, and redox status
The performance results are presented in Table 1. No significant differences (p > 0.05) were found in the performance of piglets among the four groups. The serum biochemistry results are presented in Table 2. Compared to the CON, all the serum biochemistry indices were not affected (p > 0.05) by dietary TA supplementation, while ZnO increased (p < 0.05) ALP by 34.5% and decreased (p < 0.05) TBIL by 37.3%. Compared to the ZnO group, 3.0 kg/t TA decreased (p < 0.05) the serum ALT by 52.5%, while 1.5 kg/t TA decreased (p < 0.05) the serum ALP by 22.8%. Serum antioxidant capacity analysis showed that 3.0 kg/t TA tended to increase (p = 0.054) SOD activity, while ZnO tended to reduce (p = 0.061) MDA concentrations when compared to the CON (Figure 1A,D). Meanwhile, no differences (p > 0.05) were found in SOD, GPX, and CAT activities and MDA concentrations in the serum between the TA and the ZnO group (Figure 1A–D). Further jejunum antioxidant capacity analysis showed that 3.0 kg/t TA had higher (p < 0.05) SOD, GPX, and CAT activities and lower (p < 0.05) MDA concentrations than those of the CON and/or ZnO groups (Figure 1E–H).
| CON | 1.5 kg/t TA | 3.0 kg/t TA | ZnO | p-value | |
|---|---|---|---|---|---|
| Initial BW, kg/piglet | 7.06 ± 1.19 | 7.04 ± 1.18 | 7.05 ± 1.18 | 7.05 ± 1.17 | 1.000 |
| Final BW, kg/piglet | 11.58 ± 2.19 | 12.07 ± 1.48 | 11.59 ± 1.41 | 12.19 ± 1.82 | 0.991 |
| ADFI, g/d/piglet | 298.71 ± 66.80 | 315.10 ± 36.64 | 295.93 ± 30.28 | 321.44 ± 47.02 | 0.968 |
| ADG, g/d/piglet | 215.29 ± 50.93 | 239.22 ± 23.46 | 216.08 ± 20.85 | 244.48 ± 35.07 | 0.709 |
| F/G, g/g | 1.39 ± 0.03 | 1.32 ± 0.05 | 1.37 ± 0.08 | 1.32 ± 0.07 | 0.235 |
- Abbreviations: ADFI, average daily feed intake; ADG, average daily gain; BW, body weight; F/G, feed/gain ratio.
- a Values are means ± SD, n = 6.
| CON | 1.5 kg/t TA | 3.0 kg/t TA | ZnO | p-value | |
|---|---|---|---|---|---|
| ALT, U/L | 15.29 ± 3.03ab | 18.06 ± 3.92ab | 12.85 ± 3.36b | 19.18 ± 4.41a | 0.034 |
| AST, U/L | 8.04 ± 2.61 | 10.02 ± 2.04 | 8.80 ± 2.69 | 9.63 ± 1.71 | 0.463 |
| ALP, U/dL | 30.14 ± 4.00a | 31.28 ± 3.46a | 34.75 ± 5.72ab | 40.53 ± 8.27b | 0.021 |
| TP, g/L | 50.94 ± 5.30 | 53.09 ± 8.12 | 50.43 ± 5.24 | 57.61 ± 7.49 | 0.259 |
| ALB, g/L | 33.26 ± 3.95 | 35.28 ± 1.61 | 34.46 ± 5.34 | 34.74 ± 3.80 | 0.835 |
| CREA, μmol/L | 94.87 ± 16.50 | 105.67 ± 10.62 | 103.47 ± 12.07 | 108.28 ± 12.19 | 0.337 |
| BUN, mmol/L | 1.69 ± 0.47 | 2.00 ± 0.35 | 1.73 ± 0.50 | 1.92 ± 0.21 | 0.510 |
| TBIL, μmol/L | 7.18 ± 0.79a | 6.80 ± 2.00ab | 6.61 ± 1.95ab | 4.50 ± 1.05b | 0.030 |
| GLU, mmol/L | 6.29 ± 0.72 | 6.80 ± 0.35 | 6.25 ± 0.90 | 6.27 ± 0.82 | 0.510 |
- Abbreviations: ALB, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CREA, creatinine; GLU, glucose; TBIL, total bilirubin; TP, total protein.
- * Values are means ± SD, n = 6. Labeled means in a row without a common letter differ, p < 0.05.
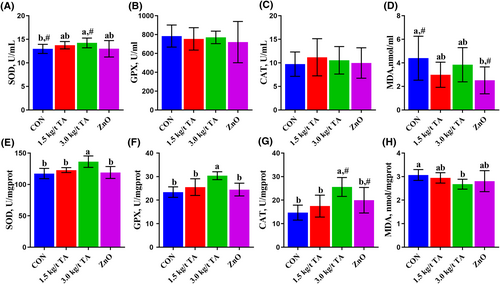
Effects of gallnut TA on the antioxidant status, including, SOD, GPX, CAT, and MDA in the serum (A–D) and the jejunum (E–H) of piglets. Data are presented as mean ± SD, n = 6. Labeled means in a row with different letters differ, p < 0.05. A given 2 means labeled with # indicates a tendency, 0.05 ≤ p < 0.10. CAT, catalase; CON, control group; GPX, glutathione peroxidase; MDA, malondialdehyde; SOD, superoxide dismutase.
Small intestinal histology
The small intestinal histopathology results are shown in Figure 2A. Compared to the CON, 3.0 kg/t TA significantly increased (p < 0.05) VH in the duodenum and the jejunum by 7.7%–25.0% (Figure 2B), while decreased (p < 0.05) CD in the jejunum and the ileum by 10.1%–11.5% (Figure 2C). Meanwhile, the higher (p < 0.05) VH:CD in the duodenum and the jejunum were found in 3.0 kg/t TA group (Figure 2D). Notably, the VH, CD, and VH:CD of the small intestine were not affected (p > 0.05) by dietary 1.5 kg/t TA supplementation when compared with CON or ZnO (Figure 2B–D). Moreover, the ZnO group only had longer (p < 0.05) VH (20.6%) and higher VH:CD (22.9%) in the duodenum than those of the CON (Figure 2B,D).
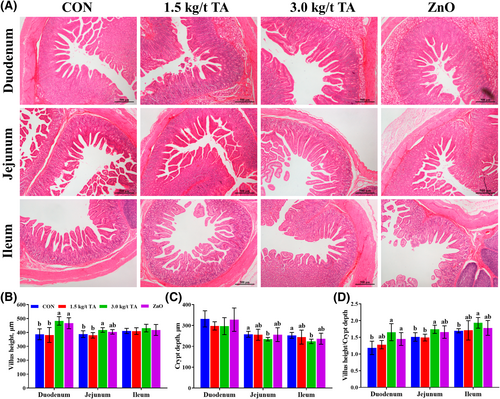
Effects of gallnut TA on the gut morphology of the small intestine of piglets. The histopathology (A) of the duodenum, jejunum, and ileum of piglets, and the morphology of VH (B), CD (C), and VH/CD (D) of the small intestine. Data are presented as mean ± SD, n = 6. Labeled means in a row with different letters differ, p < 0.05. CD, crypt depth; CON, control group; VH, villus height.
Expression of cytokine- and tight junction-related proteins
Compared to the CON, dietary supplementation of TA significantly downregulated (p < 0.05) IL-1β and TNF-α protein production, while the IL-6, Occludin, and Claudin-2 protein were not affected (p > 0.05) in the jejunum (Figure 3A–C).
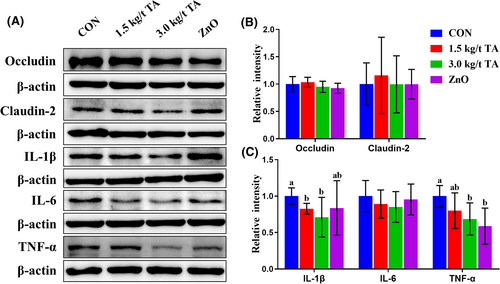
Effects of gallnut TA on protein abundance of cytokines and tight junction in the jejunum of piglets. For a given protein, each blot (A) is a representative image of 4 or 5 independent analyses, and the relative density (B and C) was shown. Values are means ± SD, n = 4–5. Labeled means without a common letter differ, p < 0.05.
Gut microbiota
Cecal chyme of a total of 24 piglets were subjected to 16S rRNA gene sequencing, and 8999 bacterial features were identified (Supporting Information S1: File 1). All the qualified sequences were then assigned to 27 known phyla and 230 genera (Supporting Information S1: Files 2 and 3). Of them, top 4 phyla and top 10 genera were predominantly identified at phylum and genus levels, respectively (Figure 4A,B). Notably, 1.5 kg/t TA had a great influence on microbiota composition at phylum level, specifically; it increased (p < 0.05) Proteobacteria abundance while decreased (p < 0.05) Firmicutes abundance when compared to CON (Figure 4A). Meanwhile, the four groups shared 4981 amplicon sequence variants (ASVs) in cecum chyme of piglets (Figure 4C). Moreover, the PCoA analysis showed significant separation in the microbial profiles of cecum (p = 0.006) among the four groups (Figure 4D). The alpha diversity results showed that the Shannon index was not affected (p > 0.05) by dietary TA supplementation, while 3 kg/t TA and ZnO reduced (p < 0.05) observed OTUs when compared to the CON (Figure 4E,F). LEfSe analysis revealed that there were 11 significant differential genera found among the groups, such as Candidatus Brocadia, Collinsella, Acetiomaculum, Escherichia-Shigella, Catenisphaera, and Acidovorax (Figure 5A). Since 3.0 kg/t TA had better gut antioxidant capacity than the CON and ZnO groups, suggesting that some specific microbial genera were altered by dietary 3.0 kg/t TA supplementation. Among these differential genera, as expected, the 3.0 kg/t TA group had lower (p < 0.05) abundance of Candidatus Brocadia, [Eubacterium] oxidoreducens group, and Escherichia-Shigella than the CON group (Figure 5B–D). Further, Spearman correlation analysis showed that the abundance of Candidatus Brocadia and Escherichia-Shigella displayed significant correlations with several redox status parameters in the jejunum (Figure 6A). Specifically, the abundance of Candidatus Brocadia had a significant negative correlation (R < −0.50, p < 0.01) with SOD and GPX activities (Figure 6B,C). Besides, the abundance of Escherichia-Shigella had a significant negative correlation (R = −0.61, p < 0.01) with CAT activity while positive correlation (R = 0.44, p < 0.05) with MDA concentrations (Figure 6D,E).
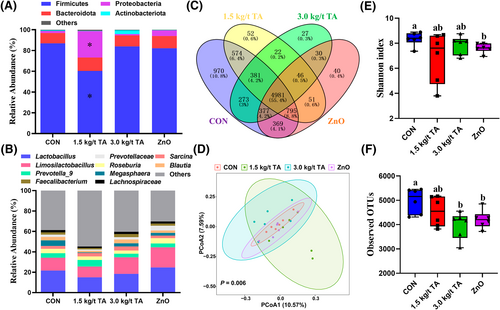
Composition and diversities of the cecal microbiota in piglets. Relative abundance of the top 4 phyla (A) and top 10 genera (B) and the number of identified ASVs (C) in the four groups. Principal coordinates analysis (PCoA) plots were generated using Jaccard distances (D). Differences in alpha diversities were calculated using the Shannon index (E) and observed OTUs (F), respectively. *indicates a significant difference (p < 0.05) between the treatment and the CON groups. Labeled means with different superscript letters are significantly different (p < 0.05) by the Kruskal–Wallis test (n = 6). ASVs, amplicon sequence variants; CON, control group.
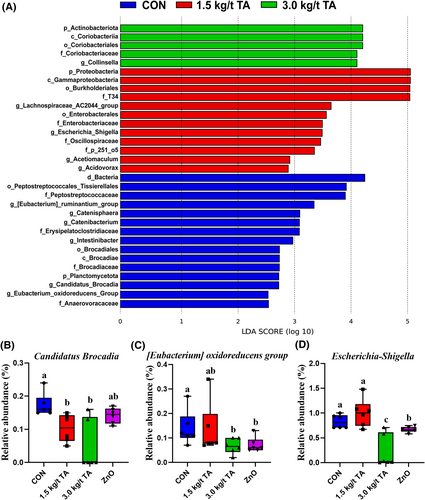
LEfSe analysis of cecal microbiota among the four groups. Differential enrichment of the bacterial taxa was identified using LEfSe with an LDA score >2.0 (A). Note that no preferential enrichment was detected in the ZnO group. Relative abundance of three differentially enriched bacterial features. Relative abundances of Candidatus Brocadia, [Eubacterium] oxidoreducens group, and Escherichia-Shigella among the four groups (B–D). Labeled means with different superscript letters are significantly different (p < 0.05) by the Kruskal–Wallis test (n = 6). LDA, Linear discriminant analysis, LEfSe, LDA effect Size.
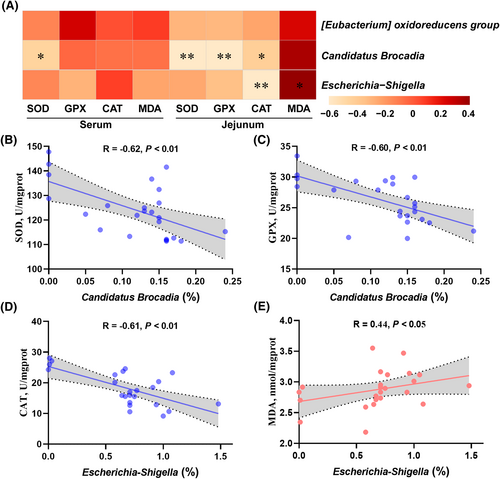
Spearman correlation between redox status (SOD, GPX, CAT, and MDA) and the differentially expressed genus (A). Scatterplots of Candidatus Brocadia and Escherichia-Shigella showing a significant correlation with redox status in the jejunum (B–E). Correlation coefficients and p-values were determined via Spearman's rho correlation test. *p < 0.05, **p < 0.01.
DISCUSSION
TA have shown to linearly reduce the growth performance of weanling pigs [29, 30]. However, many recent studies have observed that the dietary TA supplementation at an appropriate dose had no effect on growth performance and nutrient digestibility in piglets [12-15]. In agreement with this, the current study shows that dietary supplementation of 1.5 or 3.0 kg/t gallnut TA had no adverse effect on the growth performance in weanling piglets. Additionally, other studies have also found that TA can improve the growth performance of broilers and cattle [31, 32]. These inconsistent results from different trials might be owing to the source, concentration, and chemical structure of TA as well as animal species [33]. Moreover, serum biochemistry indices including activities of ALT, AST, and ALP, as well as concentrations of TP, ALB, TBIL, BUN, CREA, and GLU have been demonstrated as biomarkers for assessing the overall health status of animals [34]. These parameters were not affected by the dietary TA supplementation in this experiment when compared to the CON group. Meanwhile, dietary supplementation of 3.0 kg/t TA decreased serum ALT and ALP when compared to the ZnO group. These outcomes suggested that TA with relatively appropriate doses was nontoxic and safe to the weaned piglets, which could be a potential alternative to ZnO.
Oxidative stress can be characterized as an imbalance between a reduced activity in antioxidant enzymes and an accumulation in reactive oxygen species (ROS) [35]. Weaned piglets usually suffered severe oxidative stress due to the weak antioxidant system and the weanling stress, leading to diarrhea, organ damage, and even mortality [36, 37]. TA has been shown to be an effective antioxidant because it can combat free radicals by forming resonance-stabilized phenoxy radicals [38, 39]. Similar to the previous studies [12, 39, 40], the dietary supplementation of 3.0 kg/t TA had a better redox status, indicated by a higher SOD, GPX, and CAT activity and a lower MDA concentration in the jejunum of piglets than those of the CON or the ZnO group. The SOD, GPX, and CAT are three important antioxidant enzymes that can protect cells from damage caused by ROS overproduction, thereby alleviating oxidative stress [41]. Thus, the current results showed that dietary supplementation of 3.0 kg/t TA could effectively improve the gut antioxidant capacity of weaned piglets.
Small intestinal morphology is the most common and direct indicator of piglet intestinal development and health status [42]. It is susceptible to weanling stress, resulting in increasing permeability and impairing intestinal function [43]. Notably, dietary supplementation of 3.0 kg/t TA significantly increased villus height and villus height/crypt depth in the duodenum and the jejunum, and decreased crypt depth in the jejunum and the ileum. The results of this experiment are in agreement with previous studies [12, 44], which showed TA can improve small intestinal morphology development and enhance gut health of weaned piglets. Moreover, although the protein expression of tight junction (Occludin and Claudin-2) was not affected by dietary treatment, TA significantly downregulated pro-inflammatory cytokines (IL-1β and TNF-α) expression at the protein level in the jejunum compared to the CON. These results further suggested that TA not only does not cause damage to the intestinal barrier but may also have the potential to decelerate intestinal inflammation [45].
Gut microbiota plays a vital role in maintaining pig intestinal health [46, 47], and gut dysbiosis will lead to diarrhea and enteric infections in weanling piglets [48]. Strikingly, our results showed that 1.5 kg/t TA increased Proteobacteria abundance and decreased Firmicutes abundance, while 3.0 kg/t TA did not change their abundance compared to the CON. These results suggested that different doses of TA have different influence on Proteobacteria and Firmicutes in the cecum, which needs to be further explored. Eescherichia coli (E. coli), a member of Escherichia-Shigella, is normally a pathogen living in the intestines, which can cause diarrhea, vomiting, and cramps in piglets after weanling [49, 50]. A previous study has shown that TA can reduce the number of E. coli and post-weanling diarrhea in piglets [51], suggesting that TA has the antimicrobial property. Our results showed that the addition of 3.0 kg/t TA to the diet significantly reduced certain pathogenic bacteria, such as Candidatus Brocadia, [Eubacterium] oxidoreducens group, and Escherichia-Shigella in cecal digesta. These results also support that TA can effectively inhibit the growth of harmful bacteria and maintain intestinal microbial homeostasis in weaned piglets [16], which could also explain why TA has better intestinal morphology and lower pro-inflammatory cytokines than CON. Notably, olive extracts enriched various polyphenolic compounds (especially Hydroxytyrosol) could improve antioxidant capacity and attenuate oxidative stress by altering colonic microbiota in mice [52, 53], suggesting that gut microbiota play an important role in modulating redox homeostasis under dietary polyphenolic compounds treatment. In this study, we found similar results that TA, a natural polyphenolic compound, could also improve antioxidant capacity and modulate gut microbiota in piglets. Interestingly, our results found that the abundance of Candidatus Brocadia and Escherichia-Shigella are negatively linked to gut antioxidant capacity, suggesting that members of these two genera may play an important role in disrupting gut redox homeostasis [54]. Thus, the decrease of these two genera in the gut by dietary TA supplementation may be one of the reasons why TA could improve gut antioxidant capacity in piglets. However, the mechanism underlying TA-mediated gastrointestinal redox homeostasis by specific gut microbiota in piglets remains elusive. Future studies to separate these pure culture bacteria from piglets could further explore their function and support our findings.
The development of safe and effective alternatives to antibiotics and ZnO to improve intestinal health and attenuate weanling stress in piglets is essential for swine husbandry and food security. In this study, no difference was found in growth performance, small intestinal morphology, intestinal barrier and inflammation, serum antioxidant capacity, and cecal microbial diversity between the 3.0 kg/t TA and the ZnO group, but 3.0 kg/t TA had better jejunum antioxidant capacity than those of the ZnO group. These outcomes suggested that 3.0 kg/t TA may be a good alternative for ZnO in weaned piglets. A previous study has shown that dietary supplementation of hydrolyzed TA at 1899.5 mg/kg could be an alternative for ZnO in piglets [13], while the dose of TA is inconsistent with our results. This may take into account that hydrolyzed TA reduces the combination between TA and digestive enzymes, thereby enhancing TA bioactivities [11].
CONCLUSION
In summary, the present study revealed that the 3.0 kg/t TA supplementation can improve gut health status of piglets without altering the growth performance. The protective mechanisms of TA against weanling stress in piglets involve enhancing the redox status and morphology in the jejunum, while simultaneously regulating the composition of gut microbiota. Overall, these findings suggest that the dietary supplementation of gallnut TA at 3.0 kg/t might be a feasible nutritional strategy to improve the health of weaned piglets.
AUTHOR CONTRIBUTIONS
Zhang-Chao Deng, Jie Wang, Juan Wang, Yi-Qin Yan and Yu-Xuan Huang: Investigation, Methodology, and Data analysis; Chi-Qing Chen: Provide resources and Funding acquisition; Zhang-Chao Deng: Writing-original draft and Visualization; Meng Liu and Lv-hui Sun: Supervision, Writing-review & editing and Project administration. All authors have read and agreed to the published version of the final manuscript.
ACKNOWLEDGMENTS
This research was supported in part by the Key R&D Program of Shandong Province, China (2023TZXD038), and a research gift from Wufeng Chicheng Biotech Co., Ltd.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest to this work.
ETHICS STATEMENT
The animal protocol was approved by the Institutional Animal Care and Use Committee of Huazhong Agricultural University, China.
Open Research
DATA AVAILABILITY STATEMENT
Data are contained within the article and supplemental information files.




