4-Perchloratobutyl Acrylate, a New Monomer for Self-Crosslinking Thermosetting Acrylic Resins
Funding: The authors received no specific funding for this work.
ABSTRACT
4-Perchlorato butyl acrylate (pCBA) is prepared in a one-pot reaction and copolymerized with methyl, ethyl resp. butyl acrylate to give a polyacrylate binders with upto 11 mol% pCBA. Thermally curing in the range of 105°C–135°C gives a thermoset by a transesterification, eliminating alkyl perchlorates. The resulting 1,4-linker motive and the volatile alkyl perchlorates were identified spectroscopically (IR, TGA, (CP MAS) NMR) and validated by direct synthesis of the resin. The crosslinking process was monitored by FT IR spectroscopy (complete within an hour at 135°C) and by TGA (onset temperature of 145°C, 154°C resp. 171°C for methyl, ethyl and butyl acrylate copolymer with about 11 mol% pCBA). The crosslink density (0.15–2 mol/L) and glass transition temperatures (−12°C to 20 for copolymer of ethyl acrylate with 0.9–8 mol% of pCBA) increase proportionally to the pCBA content. Tetrahydrofuran is detected as further cleavage product, resulting from a competitive pathway leading to carboxylic acid moieties and probably crosslinks (of unknown structure).
1 Introduction
Thermosetting acrylic resins are known for their excellent outdoor durability, good adhesion properties to a manifold of substrates, and their moderate cost [1]. They are used for architectural coatings, “special purpose” coatings, and in the original equipment manufacturing market. Cured resins are more resistant to weathering (heat) and solvents than the linear or branched polymers from which they originate. The current thermosetting praxis is based on chemical reactions between entities in the side chains that lead to three-dimensional networks. This crosslinking is often accomplished by combining hydroxyl and carboxyl functional acrylic resins with a co-reacting polyfunctional polymer or hardener, often in the form of polyisocyanates, polyepoxides, or amino resins [1]. They react to give urethanes, etherified amino resins, and a smaller variety of other functionalities. These crosslinks are essential to the final film properties, and the crosslink chemistry is chosen in line with the requirements and application constraints.
Self-crosslinking polyacrylics have the advantage of needing no co-reactant and are therefore easier to handle, often are more economical and hence tend to be the first choice for manufacturers [2]. Resins with such properties generally contain a combination of functional groups that become reactive towards each other upon the action of a trigger (temperature, moisture …) and otherwise should be quite inert. The latter is important for reaching a reasonable “shelf life” that is, a period when the physical and chemical properties of the resin are not changing. The choice of reactive entities leading to crosslinking is limited, effectively to methylolated (meth)acrylamides [3], blocked isocyanates [4], alkoxysilanes [2], keto-dihydrazide systems [5], and metal complexes [6]. The crosslinking can be induced by increasing the temperature or through a reaction with water from the surrounding air. These one-component (1 K) systems have their merits, but also face criticism, for example, for the emission of volatile organic compounds or limits of shelf-lifeA possibly more important weak point may relate to the crosslinking functionality being prone to thermal or photochemical degradation.
It seemed thus of interest to develop a reactive acrylate binder that leads to a network with only ester moieties. This is probably most easily reached through a (trans)esterification of an acid-derived entity and a hydroxyl group in a side chain. However, an esterification between a free acid and a hydroxyl entity has a rather low driving force and shows low rates in an uncatalyzed execution. Similar will hold true for a transesterification involving a hydroxyl functionality [7]. These constraints must be released for an effective 1 K self-crosslinking binder system, which may not be trivial.
Herein, an unusual but simple self-crosslinking acrylic system is presented, yielding an all-ester, potentially UV-resistant coating after thermal curing. In addition, some not-so-common chemistry of covalently bonded perchlorates is described. Alkyl esters of perchloric acid are certainly versatile functional groups, and yet they have barely been applied, neither commercially nor academically. This may originate from their explosive and toxic reputation, in combination with their basically absent commercial availability and also limitations in useful precursors [8, 9]. Indeed, in particular, secondary perchlorates (e.g., sec-pentyl perchlorate) rapidly eliminate perchloric acid (HClO4) even at room temperature, and tert-butyl perchlorate is a good source of anhydrous perchloric acid [10]. However, primary alkyl perchlorate esters, for example, n-decyl perchlorate, turned out to be thermally quite stable in nonpolar solvents, up to temperatures as high as 140°C. Reasonably stable alkyl perchlorates have, in fact, been used as initiators for cationic polymerization of electron-rich alkenes [11], and ring-opening polymerization of lactones [12] and cyclic ethers [6-9, 13]. Because of the exceptionally good leaving group ability of the ClO4 − anion [14], multiple orders of magnitude stronger than mesylates and almost as strong as the esters of triflic acid, perchlorate esters are also potent alkylating agents [15]. They react not only fast with common nucleophiles like amines and alcohols [16], but even with considerably weaker ones like nitriles [17], be it at higher temperatures. Their analogous reactivity towards simple esters can be used for rearranging ester bonds in polyacrylate resins, which is reported upon here.
2 Experimental
2.1 Materials
Reactions were performed under a nitrogen atmosphere. Methyl acrylate (MA), ethyl acrylate (EA), butyl acrylate (BA), methyl methacrylate (MMA), vinyl acetate (VA) and pentane were distilled prior to use. Xylene was stored under a nitrogen atmosphere over a molecular sieve 4 Å. Acetone (technical grade), acrylic acid (Sigma Aldrich, ≥ 99%), acryloyl chloride (Sigma Aldrich, ≥ 97%), azobisisobutyronitrile (AIBN, Sigma Aldrich, 98%),1-bromodecane (Sigma Aldrich, ≥ 98%), 1,4-butanediol diacrylate (BDDA, Sigma Aldrich, technical grade), carbon tetrabromide (CBr4, Sigma Aldrich, 99%), chloroform-d1 (CDCl3, Deutero, 99.8%, +0.03% TMS), methacryloyl chloride (Merck, 97%), extra dry tetrahydrofuran (THF, Acros Organics, 99.85%) and extra dry toluene (Acros Organics, 99.85%) were used without further purification. Anhydrous silver perchlorate was purchased from Alfa Aesar, dissolved in dry toluene, and precipitated by the addition of pentane. The solvent mixture was decanted, and residual silver perchlorate was dried under reduced pressure and stored under nitrogen in the absence of light.
2.2 Characterization
FTIR measurements were performed using a Bruker Vertex 70 spectrometer in ATR-mode. Measurements were conducted in the spectral range of 4000–400 cm−1 with a resolution of 8 cm−1, averaging 18 scans per spectrum. The spectrometer was operated with the OPUS software package. Thin films of 30–60 μm were applied with a spreader on glass substrate from 40 wt.% xylene solution and cured at 105°C–135°C. The polymer was scraped off the glass substrate and placed in the spectrometer for analysis. Calibration with pristine polymers with known composition shows a linear dependence of the band maximum at ~585 cm−1 on the pCBA concentration Figure (S12).
Glass transition temperatures (T g) were measured on a Mettler Toledo equipment (DSC1). Samples of 5–15 mg were weighted into 40 μL aluminum crucibles, cold welded with aluminum foil lids, and cooled to −90°C at a rate of 20 K min−1. After they were kept constant for 3 min, the samples were heated to 350°C at a heating rate of 10 K min−1. Glass transition temperatures were determined as the center point between the tangential baseline before and after the glass transition range.
The number/weight average molecular weight (M n/M w) and polydispersity (D = M w/M n) values of the polymers were determined by size-exclusion chromatography (SEC) in THF at room temperature (flow rate = 1.0 mL min−1) on a Thermo Separation Products AS1000 auto sampler equipped with a Schambeck RI 2012 refractive index detector and a SDV (styrene-divinylbenzene) GPC linear column (5 μm). Samples were measured at a concentration of 5 mg mL−1 after filtration through a 0.45 μm pore-size membrane.
1H and 13C nuclear magnetic resonance (NMR) spectra were recorded on Bruker AV400, AV500, or AV600 MHz spectrometers in CDCl3 at room temperature, with 1H NMR data being referenced to TMS via the residual proton signals of the deuterated solvent. Solid-state 13C cross-polarization magic angle spinning (CP MAS) measurements were performed at 25°C with a Bruker Avance II 400 MHz solid-state spectrometer equipped with a 4 mm double resonance 1H/X-MAS probe head. Spectra were recorded at the resonance frequency of approximately 100 MHz. 13C Chemical shifts were calibrated through the tertiary carbon resonance of alanine as an external reference at 51 ppm. Polymers were cooled with liquid nitrogen and ground to a fine powder before measurements.
The thermal properties and the detection of the emerging gases, which are produced when the samples were heated, were measured by means of (a) TGA-IR with a thermobalance (STA 409C/CD from Netzsch) coupled with a Fourier Transform InfraRed Spectrometer (FT-IR TENSOR 27 with external gas cell from Bruker) in 0.3 mL Al2O3 − DTA/TG-crucibles. The samples were measured under a nitrogen (5.0) flow (50 mL/min) from room temperature to 900°C with a heating rate of 10 K min−1. (b) TGA-MS with a thermobalance (TG 209 F1 Libra, Netzsch) in the dynamic mode with a heating rate of 10 K min−1 under nitrogen (5.0) flow (20 mL min−1) from room temperature to 900°C. Evolving gases were analyzed by a coupled quadrupole mass spectrometer (QMS 403 D Aëolos, Netzsch) equipped with electron ionization and a Faraday detector. Gases were transferred via a heated adapter (200°C) and through a heated quartz glass capillary (200°C) connecting the TGA furnace outlet with the MS gas inlet.
Trapping of evaporating compounds from heated polymers was performed by connecting a 10 mL Schlenk flask loaded with 200 mg of polymer through a 5 cm tube to a cold trap. After 30 min of full vacuum, the cold trap was dipped into liquid nitrogen and the valve of the flask was slightly opened to assure a nitrogen stream from the flask through the cold trap. The resin was heated at a rate of approximately 10 K min−1 to a final temperature of 200°C. The trapped compounds were analyzed by NMR-spectroscopy.
The crosslink density (CD) and number average molecular weight between crosslinks, M c, were calculated using the Flory-Rehner equation: ln(1 − V 2) + V 2 + χV 2 2 = (−ρV 1/M c)(V 2 1/3 0.5V 2), where V 2 is the volume fraction of the polymer in the swollen gel at equilibrium, χ is the polymer-solvent interaction parameter, ρ is the polymer density, and V 1 is the molar volume of the solvent. For the system PEA-PCBA/acetone, the equation can be written as: M c = −1.12 g cm−3 * 106.26 cm3 mol−1 (V 2 1/3 0.5 V 2)/ln(1 − V 2) + V 2 + 0.36 V 2 2. For simplification, ρ was set to the density of the base polymer (PEA). χ = 0.36 was calculated using the Bristow and Watson semi-empirical equation, Χ = β 1 + (V 1/RT) (δ s − δ p)2, where β 1 is the lattice constant (0.34), R is the universal gas constant, T is absolute temperature, and δ s and δ p are the Hansen solubility parameters of the solvent and polymer, respectively.
Acetone was selected as a swelling solvent over toluene, as its Hansen solubility parameter (19.9 Mpa1/2) is in between that of PEA (19.1 Mpa1/2) and PAA (23.3 Mpa1/2). The substantial change in polarity of the base polymer by the formation of COOH groups, especially for high ClO4-content formulations, is better balanced by acetone than by for example, toluene (18.3 Mpa1/2), in which some highly crosslinked films did not swell, complicating further analysis.
The volume fraction of the polymer was calculated by a method reported by Hill [18]. Films of 30–50 μm thickness were applied on PTFE/glass fiber composite sheets or glass substrates and cured at 105°C or 135°C. Round platelets of 1 mm diameter were punched out using a hollow puncher, placed onto a microscope slide, and covered with a thin coverslip. The diameter of the unswollen sample was measured with a microscope equipped with a digital measuring device set at 2.5 magnification. A few solvent drops were placed at the slide/coverslip interface, whereupon the solvent was drawn into the small gap between the two glasses. As soon as the samples came in contact with the solvent, they began to swell until an equilibrium was reached, usually within 2 min. The fractional increase in diameter (f) was calculated as f = (x 2 − x 1)/x 1, where x 1 is the diameter of the sample before swelling and x 2 is the diameter of the sample in the equilibrium swollen state. V 2 is calculated as V 2 = 1/(1 − f)3, assuming isometric swelling.
CD was derived from M c and the density of PEA (1.12 g cm−3): CD (mol cm−3) = 1.12 g cm−3 M c −1. Each data point is the average of six diameter measurements from 3 individual samples. The calculated CD is taken from M c,theo = M EA (100 mol%)/2X, where M EA is the molecular weight of ethyl acrylate, and X is the molar ratio of pCBA in the polymer in percent.
2.3 Synthesis of Monomers and Polymers
2.3.1 Synthesis of 4-Perchloratobutyl Acrylate ( 3a , pCBA)
Silver perchlorate (0.85 g, 3.7 mmol, 1.3 eq.) was suspended in pentane (24 mL) and cooled to −20°C. Under vigorous stirring, acryloyl chloride (0.24 mL, 2.9 mmol, 1 eq.) was added, and the reaction mixture was allowed to warm to room temperature. The suspension was stirred for 1 h, and brownish silver chloride precipitated. The mixture was again cooled to −20°C before THF (0.24 mL, 2.9 mmol, 1 eq.) in pentane (2 mL) was added dropwise, and the reaction mixture was subsequently stirred for another hour at room temperature. The formed salt was removed by filtration through a pad of silica gel, and the resulting clear solution was dried over molecular sieve 4 Å for at least 12 h before further use. The solution was again filtered, and the solvent was removed under reduced pressure. pCBA is obtained as a clear liquid in 50%–80% yield. It is water sensitive and was stored in a solution of pentane under nitrogen atmosphere at −36°C. It does not explode in the hammer test [19].
1H NMR (600 MHz, CDCl3, δ): 6.39 (d, J = 18.2 Hz, 1H), 6.10 (dd, J = 17.3 Hz, 1H), 5.83 (d, J = 10.4 Hz, 1H), 4.57 (t, J = 6.3 Hz, 2H), 4.19 (t, J = 6.2 Hz, 2H), 1.95–1.87 (m, 2H), 1.85–1.78 (m, 2H). 13C NMR (150 MHz, CDCl3, δ): 166.1, 131.1, 128.2, 75.5, 63.4, 24.7, 24.4. IR (cm−1) ν = 2966, 1721, 1637, 1409, 1296, 1222, 1185, 1064, 1032, 984, 903, 810, 702, 623, 579. EA calc. for C7H11ClO6 (226.02): C 37.10, H 4.89, O 42.36; Found: C 37.33, H 4.91, O 42.20.
2.3.2 Synthesis of 4-Perchloratobutyl Methacrylate ( 3b , pCBMA)
Silver perchlorate (0.85 g, 3.7 mmol, 1.3 eq.) was suspended in pentane (24 mL) and cooled to −20°C. Under vigorous stirring, methacryloyl chloride (0.24 mL, 2.9 mmol, 1 eq.) was added, and the reaction mixture was allowed to warm to room temperature. The suspension was stirred for 1 h and brownish silver chloride precipitated. The mixture was again cooled to −20°C before THF (0.24 mL, 2.9 mmol, 1 eq.) in 2 mL of pentane was added dropwise. The reaction mixture was subsequently stirred for another hour at room temperature. The salt was removed by filtration through a pad of silica gel, and the resulting clear solution was dried over molecular sieve 4 Å for at least 12 h before further use. The solution was again filtered, and the solvent was removed under reduced pressure. pCBMA was obtained as a clear liquid in 30% yield. It is reactive towards water and was stored in a solution of pentane under nitrogen atmosphere at −36°C. It does not explode in the hammer test [19].
1H NMR (600 MHz, CDCl3, δ): 6.09 (s, 1H), 5.57 (t, J = 1.5 Hz, 1H), 4.59 (t, J = 6.3 Hz, 2H), 4.19 (t, J = 6.2 Hz, 2H), 1.95–1.89 (m, 5H), 1.86–1.80 (m, 2H). 13C NMR (150 MHz, CDCl3, δ): 167.4, 136.2, 125.8, 75.5, 63.6, 24.8, 24.5, 18.3. IR (ATR) ν = 2963, 1715, 1637, 1404, 1296, 1224, 1160, 1032, 914, 814, 703, 625, 579 cm−1. EA calc. for C8H13ClO6 (240.04): C 39.93, H 5.45, O 39.89; Found: C 43.29, H 5.57, O 38.46.
2.3.3 General Procedure of Acrylate Polymerization
A solution of (meth)acrylate (1.4 mL), pCBA, azobisisobutyronitrile (AIBN, 40 mg, 0.25 mmol) in toluene (6.5 mL) was purged for 30 min with nitrogen and subsequently heated to 60°C for 30 min. The mixture was allowed to cool to room temperature and poured into cold pentane. The overlaying solution was discarded, polymers were redissolved in dichloromethane, and the procedure was repeated one more time. The precipitated polymers were dried under reduced pressure until a constant weight was attained.
3 Results
Covalently bonded perchlorates in (acrylic) polymers appear to be unknown; only polymers with integrated perchlorates in the form of salts are described [20]. A perchlorate-containing resin could be prepared from 4-perchloratobutyl acrylate (pCBA), an acrylic monomer carrying the perchlorate ester functionality. It is obtained from acryloyl chloride, silver perchlorate, and tetrahydrofuran (Scheme 1). Treatment of acryloyl chloride with silver perchlorate in a nonpolar solvent like pentane immediately gives a brownish precipitate (of AgCl) indicative of the formation of acryloyl perchlorate, a highly reactive species readily attacked by any nucleophile. Subsequent addition of THF yields an oxonium ion (2), which precipitates as a white salt and then further converts to pCBA by effectively adding the perchlorate anion to the electrophilic (alpha) carbon atom adjacent to the oxygen. The formation of a minor amount of polyTHF, as was reported under similar conditions [21, 22], cannot fully be avoided in pentane, even when adding THF as a solution in pentane. However, the insoluble, precipitating polyTHF is readily removed by filtration through a small pad of silica gel, and solvent removal under reduced pressure leaves the essential pure liquid monomer in up to 80% yield behind. The more polar carbon tetrachloride may be a good choice for a solvent too (instead of pentane), but was not tried here; the analogous reaction of equimolar amounts of THF and acetyl perchlorate was reported to yield almost quantitatively the perchlorate ester [23].
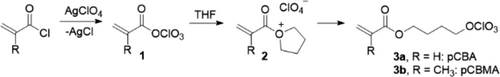
pCBA is moisture sensitive but can be stored as a solution in pentane at room temperature for some days. The identity was established from 1H, 13C NMR, and IR spectra and by elemental analysis. Note that silver perchlorate is explosive and should be handled accordingly in limited amounts and with appropriate precautions. pCBA, in contrast, did not explode in the hammer test [18]. The corresponding methacrylate can be prepared under the same conditions, giving 4-perchlorato butyl methacrylate (3b; pCBMA).
Free radical (co)polymerizations of pCBA in toluene solution are straightforward and yield linear polymers. Bulk polymerizations were not attempted but may be possible at low temperatures. Copolymerization with various acrylates, methacrylates, or vinyl acetate proceeds smoothly at 60°C (60 wt.% in toluene). Polymerizations at temperatures above 100°C lead to significant crosslinking. The copolymerization reactivity ratio of pCBA is, as expected, approximately equal to other short-chain alkyl acrylates, and statistical random copolymers are obtained.
The molecular weights of the copolymers with the perchlorate entity in the side chain are lower than those of homopolyacrylates prepared from alkyl acrylates under the same conditions (Table S1). The free radical polymerization of ethyl acrylate (EA) in the presence of 1 mol% n-decyl perchlorate (relative to EA) resulted, for example, in a polyethylacrylate PEA with M n of 56 kDa and a broader molecular weight distribution (PDI of 2.7) than homo-PEA (M n of 66 kDa; PDI of 2.1) obtained similarly in its absence. The perchlorate esters thus show some chain regulating activity. The underlying reactions are not understood and would need more attention. Chain regulation with carbon tetrabromide in the free radical copolymerization with pCBA gives access to polymers of lower molecular weight (M n < 10,000 g mol−1); mercaptan regulators are too nucleophilic to be used in combination with perchlorate esters.
The obtained polyacrylic resins from pCBA and alkyl acrylates are readily crosslinked with a variety of typical hardeners with hydroxyl and/or amine moieties (results not shown), but these reagents are not necessary for crosslinking the binder. As indicated above, it was unexpectedly found that a simple thermal treatment is sufficient to reach an all-ester (robust) crosslinked coating. Heating thin films of EA/pCBA copolymers over 100°C leads to a thermoset as indicated by swelling studies [19]. The crosslink density (CD) increases for about 4 h while curing at 105°C to reach a constant level for copolymer compositions with less than 8 mol% of pCBA (highest level studied). Curing at 135°C takes a similar course, reaching completion after 1 h (Figure 1a,b).
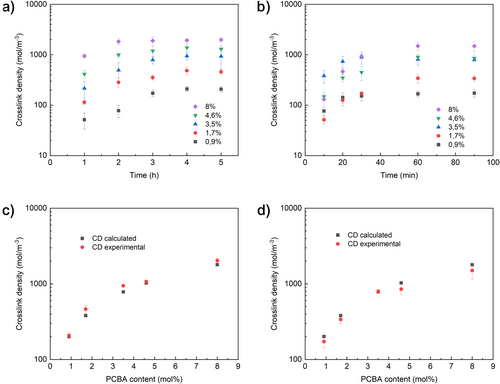
The experimental CDs are generally in good alignment with CDs that were calculated assuming that all perchlorate entities lead to a crosslink (Figure 1c,d). The crosslinking seems thus quite effective; every mol of incorporated pCBA generates about 1 mol of crosslinks. The glass transition temperatures (T g) of completely cured resins (Figure S1) were found to increase with increasing pCBA content, as a higher level of crosslinking shortens segments and impedes general mobility. Linear PEA shows a T g of −23°C; cured P(EA-pCBA0.9–8) has a T g between −12°C and +20°C (note that alkyl acrylate copolymers containing x mol% of pCBA are designated P(YA-pCBAx), with x the mol% of pCBA in the backbone and Y being either M for methyl, E for ethyl and B for butyl acrylate content).
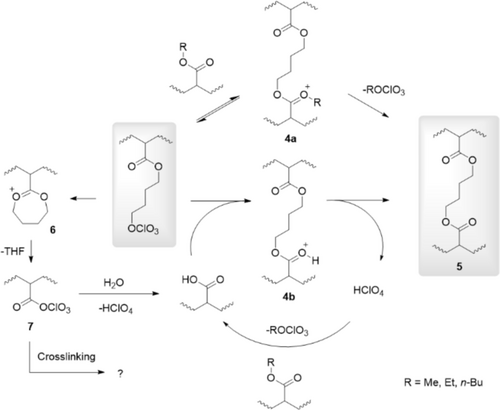
The mechanism of crosslink formation is envisioned to proceed by the nucleophilic attack of an ester entity at the electrophilic carbon of the perchlorate ester, generating a cationic 1,3-dioxolenium intermediate 4a (Scheme 2). Subsequent liberation of one of the alkyl groups in reaction with the “free” perchlorate entity will regenerate a perchlorate ester, either in the form of the starting material or in the form of a mobile and here also volatile alkyl perchlorate. The equilibrium will shift to the side of the 1,4-butylene ester crosslink 5 when the (volatile) alkyl perchlorate is dynamically removed from the film.
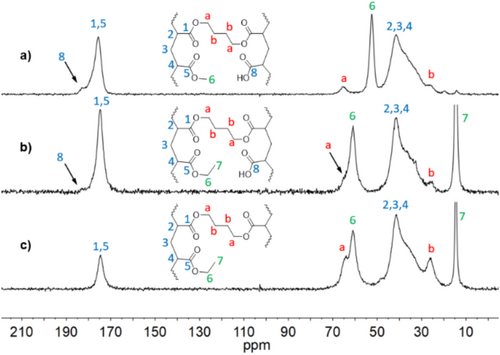
Accordingly, the 13C CP MAS NMR spectra of cured films of P(MA-pCBA11) and P(EA-pCBA8) fail to show a signal for covalently bonded perchlorate entities, expected at ~75 ppm. Therefore, new resonances appear at 25, 65, and 181 ppm (Figure 2a,b) [24]. The signals at 25 ppm and 65 ppm are related to the newly established ester crosslink and are assigned to a symmetrical 1,4-butylene unit in between two carboxylic ester moieties. These resonances are also present in the corresponding spectra of independently prepared poly(ethyl acrylate-co-1,4-butanediol diacrylate) resin (Figure 2c).
Also, a set of minor reactions was identified to take place during the curing. The intramolecular variant of the transesterification process, the nucleophilic attack of the butyl perchlorate carrying ester, will regenerate a coordinated THF-oxonium ion 6, and subsequently, THF and a polymer-bound analog of 1, acyl perchlorate 7, are expected to be formed [21, 25]. The reaction of 7 with water results in the formation of a carboxylic acid substituent and perchloric acid. The resonance at 181 ppm likely originates from free carboxylic acids formed by partial hydrolysis in the film (from the air during curing). Further evidence for the presence of carboxylic acids was found in IR spectra of cured polymers with emerging bands at ~3280 cm−1 (broad) and ~ 1715 cm−1 (Figure 3). Spectra of P(MA-pCBA11) cured in the absence of water do not show these features (Figure S2). It may be expected that the formed carboxylic acids are even more favorable for crosslink formation, as they are more nucleophilic than the corresponding esters. In addition, the presence of acid functionalities in the final polymer can be advantageous as they increase the adhesion of the resin to metals and glass substrates. Some moisture can thus be tolerated in the perchlorate ester-based crosslinking chemistry.
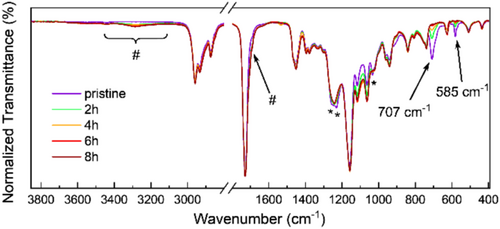
The curing process is conveniently monitored by ATR FT-IR spectroscopy of the films (Figure 3). The solitary band at around 585 and 707 cm−1 of the perchlorate anion in the spectra of the copolymers is a good probe to detect changes related to the perchlorate ester. They show the same changes as the characteristic absorptions of covalently bonded perchlorates [8] at around 1260, 1230, and a broad signal from 1100 to 1000 cm−1, but lack the partly strong overlap with other absorptions. The signal maxima show an approximate linear dependence on the pCBA concentration in the virgin polymers up to 10 mol% (using the CO vibration at ~1700 cm−1 as an internal standard).
Heating of P(BA-pCBA10) thin films at 105°C leads to a loss of perchlorate ester entities in a period of about 8 h. The loss has approximately a first-order dependence on the perchlorate concentration. Similar results are found for curing at 115°C resp. 125°C but at higher rates (Figure S3). Other aliphatic acrylic copolyesters with pCBA show this behavior too, with increasing rates in the order of BA, EA, and MA Figure (S4). The perchlorate entities disappear from the films in the form of volatile perchlorate ester alkyl derivatives and not by decomposition. This is in accordance with the thermal stability of primary alkyl perchlorates; some have been distilled at temperatures above 100°C [26]. As mentioned above, it was found that n-decyl perchlorate is stable in 1,1,2,2-tetrachloroethane at 140°C for several hours.
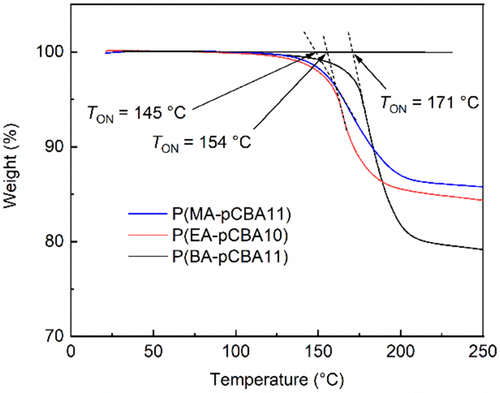
Thermographic analyses (TGA FT-IR) of copolymers of pCBA with MA, EA, or BA reveal a two-stage loss of mass, starting at around 140°C and extending to 220°C (Figure 4) and at 300°C to 500°C when heated at 10 K min−1 under nitrogen (Figure S5). The decomposition at the high-temperature range is the general thermal decomposition of polyacrylates [27]. The loss of mass starting at about 140°C is consistent with the loss of alkyl perchlorates from the samples. The onset temperatures (T ON) have the same order as the rate of perchlorate loss from the films: 145°C for P(MA-pCBA11), 154°C for P(EA-pCBA10), and 171°C for P(BA-pCBA11) (Figure 4).
The loss of mass is higher than calculated, presuming solely alkyl perchlorate loss from the film (Table 1; Figure S5). It correlates, as expected, with the pCBA content (Figure S6) and with the mass of the side chain in the corresponding comonomer. The superseding loss of mass has a proportionality to the (molecular) mass of the comonomer and also to the onset temperature. However, overall, the feature is not well understood: it indicates that some polymer backbone decomposition takes place, but little evidence is found.
| Comonomer | pCBA-content (mol%) | Expected loss of weight (%) a | Actual loss of weight (%) |
|---|---|---|---|
| MA | 11 | 12.4 | 13 |
| EA | 10 | 11.4 | 15 |
| BA | 11 | 12.4 | 20 |
- a For the elimination of the respective alkyl perchlorate while forming a butylene crosslink 5.
The IR spectra of the off-gasses are coincident with that of a mixture of the respective alkyl perchlorates, THF, and CO2 (Figure 5). In addition, products like alcohols and some ethers were detected. The formation of the mixture of alkyl perchlorates and THF was corroborated by analyzing the volatiles from P(EA-pCBA10) and P(BA-pCBA11) while heating to 200°C under a dynamic vacuum and collecting the volatiles in a liquid nitrogen trap. 1H NMR spectra of the liquids indicate the presence of ethyl perchlorate or butyl perchlorate, respectively, and some THF (Figure S7). The extent of THF formation (i.e., isolation from the trap), however, is not enough to account for the missing mass. CO2 may (in part) originate from partial decomposition of gaseous alkyl perchlorates in the heated transfer line (200°C) connecting the TG unit and the IR instrument. Separate thermogravimetric analysis-mass spectrometry (TGA-MS) on benzene with a mixture of benzene and ethyl perchlorate gave similar results. This observed reactivity of ethyl perchlorate (and other low molecular weight alkyl perchlorates) under such conditions is consistent with prior reporting [26, 29]. The decomposition of ethyl perchlorate C2H5O4Cl yields CO2, dihydrogen, and HCl in the appropriate stoichiometry. Additional calorimetric data obtained from differential scanning calorimetry (DSC) are in alignment with the thermogravimetric data and reveal an exothermic peak in the relevant temperature range of 140°C–230°C (Figure S8).
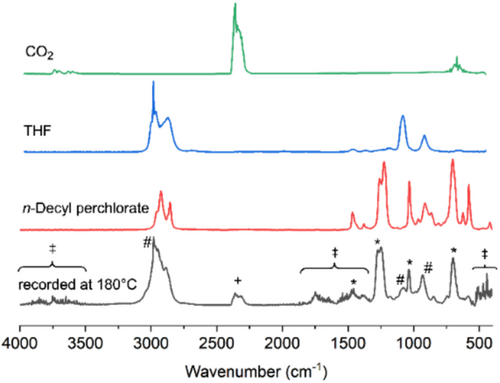
As ethyl perchlorate is the only perchlorate ester identified when P(EA-pCBA) is cured, it is concluded that the ethyl entity must originate from ethyl acrylate units. The curing mechanism thus involves a transesterification substituting the butyl perchlorate for an ethyl perchlorate. THF will originate from the butyl pCBA units (Scheme 2). The formation of THF in a side reaction would go along with the formation of a net equivalent of a perchlorate anhydride 7 resp. of a consecutive product (Scheme 1). Swelling measurements suggest, however, that 7 ultimately forms a crosslink. The reaction of 7 with an ester entity could lead to a bridging anhydride and an alkyl perchlorate. The putative formation of an anhydride, however, could be ruled out. The 13C NMR spectrum of a prepared poly(ethyl acrylate-co-acrylic anhydride) was not coincident with that of the cured product. Alternatively, a formed perchloric acid (e.g., after reaction of 7 with water) may either also evaporate from the film or, more likely, react in an equilibrium reaction with ester entities under the formation of an alkyl perchlorate and a further carboxylic acid [30]. The generated free carboxylic acid would then readily be alkylated by perchlorate esters, as demonstrated in model reactions (also leading to free perchloric acid). A crosslink is again generated in such a case when an intermediate 1,4-bis-perchlorato butane would be involved. The intermediate formation of 1,4-bisperchlorato butane can be envisioned along the same route that leads to crosslink motive 5 (Scheme 2). If such a crosslinking proceeds between two pCBA units, the outcome is 5 and 1,4-bis-perchlorato butane. The latter would also be able to regenerate a pCBA unit under the elimination of an alkyl perchlorate. Such a process also distributes the crosslinks dynamically over the film.
The importance of reactions that are coupled to the formation (elimination) of THF, which we expect to be the initiating cause of the superseding loss of mass (faster at higher temperatures) however, remains unclear at this moment. It may at least be concluded that the initial alkylation to intermediates 4a and 6 represents the key steps in the crosslink formation as THF and alkyl perchlorate are liberated simultaneously from the heated polymer despite having considerably different boiling points (Figures S9–S11). The onset of curing is higher for longer alkyl chains, indicating that the reaction leading to 4a is slower with sterically larger entities on the ester moieties, making the formation of 6 more competitive and thus inducing the partial breakdown of the binder (Figure S4).
4 Conclusion
Alkyl esters of perchloric acid were incorporated in poly(meth)acrylates by copolymerization with 4-perchloratobutyl (meth)acrylate (pCBA/pCBMA). Polymers were found to self-crosslink upon heating between 105°C and 135°C, giving a thermoset material. NMR studies revealed that perchlorate esters react with carboxylic esters in a transesterification to form a new type of crosslink in 1 K curable binders, a butylene unit connecting two carboxylic esters. TGA-FT-IR measurements confirmed that volatile alkyl perchlorates by-products formed during the transesterification quickly evaporate from the polymer surface, effectively shifting the equilibrium to the product side. Some THF was also detected leaving the polymer film, which suggests that additional reactions take place, leading to carboxylic acids and possibly to crosslinks (of unknown structure). The butylene crosslink motive 5 constitutes a nonpolar crosslink that does not provide a target for chemical attack as its esters do not differ from the esters of the acrylic backbone, and unless esters are cleaved, the butylene crosslink remains intact. Such a nonpolar crosslink, even though its formation comes with some downsides related to the perchlorate, constitutes a unique crosslink that stands out in the field of self-crosslinking acrylic resins. It opens up opportunities for application in the high-end coatings industry where robust polyacrylates are required. Of course, the potential hazards that come along with volatile alkyl perchlorates may be an obstacle for wide application. A desirable catalytic variant would have the option of minimizing such risk; this is under development.
Author Contributions
G. A. Luinstra: conceptualization (lead), formal analysis (supporting), resources (lead), supervision (lead), writing – review and editing (lead). P. Wienefeld: conceptualization (supporting), data curation (lead), formal analysis (lead), investigation (lead), methodology (lead), validation (lead), visualization (lead), writing – original draft (lead), writing – review and editing (supporting).
Acknowledgments
We would like to thank Prof. Dr. M. Fröba for providing access to the TGA-IR instrument and Uta Sazama for carrying out the measurements. Open Access funding enabled and organized by Projekt DEAL.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.




