Internal topology and water transport in tetrafunctional epoxy resins
Funding information: AkzoNobel; Engineering and Physical Sciences Research Council, Grant/Award Number: EP/S004963/1
Abstract
Water sorption into epoxy resins limits the performance lifetime of many composites, adhesives, and coatings. One hypothesis for the well-known hygroscopicity of epoxies is that the well-known internal nodular topology of epoxy networks enhances permeability. This theory has however remained untested to date, since previous attempts to manipulate the internal topology have been accompanied by changes in the hydrogen bonding capability of resins, which dominates water uptake. Here, the nanostructure of highly cross-linked network polymers based on tetra glycidyl diaminodiphenyl methane is varied in the absence of significant polarity effects by careful optimization of the cure schedule. The internal morphology of resins cured at 100, 150, and 200°C are characterized using Peakforce tapping mode atomic force microscopy, whilst the cure progression at each temperature is analyzed using in-situ transmission mode infrared spectroscopy. Distinct nodular morphologies are found to form at high cure temperatures (150 and 200°C), where spectroscopic analysis demonstrates that excess epoxy consumption occurs prior to the gel point, as a result of intra-cluster cross-linking and reduced reaction selectivity. Surprisingly, the establishment of an internal nanostructure is, however, found to have negligible effect on the extent and kinetics of moisture sorption.
1 INTRODUCTION
Highly cross-linked network polymers based on tetra glycidyl diaminodiphenyl methane (TGDDM) are known to confer excellent mechanical properties, chemical resistance, and thermal stability to structural adhesives and fiber composites used in the aerospace industry.1, 2 Nonetheless, extensive environmental water sorption into these network polymers is known to accelerate oxidative degradation and induce swelling, cracking, and plasticisation.3-8 The elimination of extensive water uptake into these polymers is thus a long-standing goal of materials scientists, however, generally, epoxy resins are well-established to be hygroscopic materials, capable of adsorbing 2%–5% water upon immersion.9-12
One potential underlying source of hygroscopicity in highly cross-linked epoxy resin materials is their recently confirmed internal nanostructure.13-15 This is comprised of heterogeneously cross-linked nodular domains, which are expected to provide low energy pathways, giving a structural basis for the relatively low fracture toughness of epoxy network materials, and their high permeability.16-18 Previously, Sahagun et al. showed that for epoxy-amine polymers the cure temperature and stoichiometry determine the extent of internal heterogeneity, and it was proposed that the relative kinetics of primary (chain extension) and secondary (cross-linking) amine reactions determined the size of the nodular domains formed.18 In support of this, we have recently demonstrated that the formation of nodular features in epoxy-phenolic resins is dependent on the reaction selectivity and overall cross-linking density.14, 15 In addition, molecular dynamic simulations have indicated that higher functionality components are expected to yield larger pre-gelation clusters, which are considered to be the precursors to nodular domains.19 Thus, any influence of nanostructure may be postulated to be particularly significant for highly cross-linked networks, such as those based on TGDDM.
The hypothesis that nanostructure plays a significant role in water transport is however, largely untested. We recently demonstrated that that moisture uptake is indeed heterogeneous at the nanoscale in epoxy-phenolic resins, and that heterogeneous nanostructure appears to be a near ubiquitous feature of even lightly cross-linked epoxy resins.14, 20-22 However, given the limited understanding of nanostructure development, attempts to control the formation of nodular topology in epoxy resins have, to date, necessitated significant changes to the overall cure degree or the chemical structure of resin components. Isolating the effect of nanostructure on water transport is thus problematic, since an extensive literature examining water uptake into epoxy resins has identified that equilibrium water uptake is primarily determined by the hydrogen-bonding capability of the network, (i.e., by the chemical structure of the epoxy and cross-linker), and, as a secondary factor, the available unoccupied volume (free volume).10, 23-28 Indeed, initial attempts to isolate the effect of nanostructure on transport properties in chemically similar epoxy-phenolic coatings were recently reported using gravimetric and electrochemical techniques, however the large disparity in cross-link density used to control nanostructure dominated water sorption and resistivity properties.14
In this contribution, we investigate the effect of internal nanostructure on water sorption using highly cross-linked network polymers comprised of TGDDM and a trifunctional phenol. In order to mitigate the effects of cross-link density and polarity, identical stoichiometric formulations are employed, and the internal topology is varied using only cure schedules, identified using a combination of Peakforce AFM analysis and in-situ infrared spectroscopy. Furthermore, the selection of cross-linker allows direct comparison to the recently reported heterogeneous nanostructure of more lightly cross-linked resins based on diglycidyl ether of bisphenol-A, prepared using the same 1,1,1-tris(4-hydroxyphenyl)ethane curing agent. Finally, water sorption is here precisely assessed, using dynamic vapor sorption analysis (DVS).
2 EXPERIMENTAL
2.1 Materials
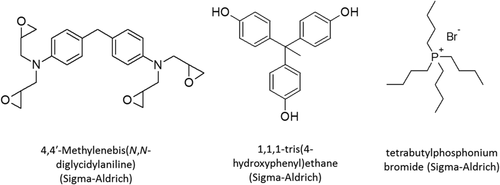
4,4′-Methylenebis(N,N-diglycidylaniline) (≤100%), 1,1,1-tris(4-hydroxyphenyl)ethane (99%) and tetrabutylphosphonium bromide (98%) were purchased from Sigma-Aldrich and used as received.
2.2 Sample preparation
Epoxy-phenolic formulations were prepared by mixing 3 mmol 4,4′-Methylenebis(N,N-diglycidylaniline) with 4 mmol 1,1,1-tris(4-hydroxyphenyl)ethane in 3 mL acetone in the presence of 0.12 mmol tetrabutylphosphonium bromide catalyst (Scheme 1). Catalytic content was chosen on the basis of previous works.13-15 The mixture was spread onto PTFE sheet (Polyflon), then cured in a pre-heated fan oven at 100, 150, or 200°C for the prescribed curing time (3–60 min). Specimens were then removed from the oven to a room temperature environment (22–24°C), removed from the PTFE sheet and stored in a desiccator.
2.3 Characterization techniques
Transmittance Fourier transform infrared (FTIR) spectra of samples were obtained using a FTIR-spectrometer (Nicolet 5700 spectrometer, Thermo Electron Corp.) equipped with room-temperature DTGS (deuterated triglycine sulfate) detector operating at 4 cm−1 resolution across the 4000–500 cm−1 range. For in-situ measurements, an open cell heated transmission system was used. Prior to experiments, the temperature at the surface of the KBr disc was adjusted to 100, 150, or 200°C using a k-type thermocouple and an automatic temperature controller (Graseby Specac). After collection of a background, freshly prepared epoxy-phenolic mixture was applied to the heated KBr disk and spectra were gathered continuously for 120 min. 64 co-averages were added to every spectrum. For epoxy consumption calculations, a spectrum of the epoxy-phenolic mixture was obtained in an identical manner on a KBr disk at ambient temperature.
The internal nanostructure of resins was assessed using atomic force microscopy (AFM) images (Multimode 8, Bruker, Santa Barbara). Samples were cryogenically fractured immediately prior to analysis. Images were collected in Peakforce tapping mode using a Pt-Ir coated probe (nominal spring constant 2 N/m, nominal resonant frequency of 80 kHz, Bruker).
Gel points were estimated by preparing a series of specimens cured at 1–5 min intervals, where gelation was assumed to occur when complete dissolution of the specimen no longer occurred (in acetone, after sonic mixing for 20 min at 60°C).
For differential scanning calorimetry (DSC) measurements, 3–10 mg of the specimens was placed in closed hermetic aluminium pans. For Tg analysis, DSC thermograms were obtained in heat-cool-heat mode over a temperature range of 0 to 200°C under nitrogen, using a heating of 10°C min−1 and a cooling rate of 5°C min−1 (Q100 DSC, TA Instruments). Tg values were calculated using the second heat trace to eliminate effects of physical aging. To assess enthalpic recovery, thermograms were obtained in modulated DSC mode, over a temperature range of −90 to 300°C under nitrogen, using a heating/cooling rate of 1°C min−1 with a modulation period of ±0.24°C min−1.
Dynamic vapor sorption was performed using a DVS 2085 (Surface Measurement Systems). Specimens comprised of free-standing weighing 0.07–0.12 g were exposed to 2000 min cycles of 0.1% RH and 90% RH at 30°C and the resulting changes in mass were continuously monitored.
3 RESULTS AND DISCUSSION
3.1 Atomic force microscopy
Three different cure temperatures were utilized in the present work: 100, 150, and 200°C. Since it has been shown that the nanostructure of resins is established at gel point and remains unchanged upon further curing,13 for nanostructural investigations all specimens were cured for 60 min to ensure a complete reaction. In keeping with previous reports, the internal nanostructure of epoxy-phenolic resins was then assessed using peak force tapping mode AFM imaging of the interface produced by fracturing under liquid nitrogen, Figure 1.13-15 This approach has previously been shown to deliver detailed morphological information, confirmed by electron microscopy.13

The fracture interfaces of specimens cured at 100°C were consistently found to be less rough than those cured at higher temperatures (Ra values of 1.27, 3.19, and 8.59 nm for 2 × 2 μm images for resins cured at 100, 150, and 200°C, respectively). Moreover, for resins produced by curing at 150 and 200°C, high resolution images of the fracture interface display well-defined nodular morphologies, with comparable dimensions (<50 nm diameter) to those previously reported for DGEBA based epoxy – 1,1,1-tris(4-hydroxyphenyl)ethane resins.15 Note that whilst nodule domains do not appear larger in the case of highly cross-linked TGDDM resins, this may be due to the known difficulty in precise analysis of nodule dimensions comparable to the AFM probe tip radius;29 that is, the resolution limits of AFM imaging. The temperature dependence of nodule development is, however, in keeping with the reported evolution of nanostructure in lightly cross-linked epoxy-amine resins, which Sahagun et al found to be a result of increased pre-gelation intra-domain cross-linking at higher temperatures.18 The applicability of this mechanism to the highly cross-linked epoxy-phenolic resins was therefore investigated using in situ FTIR analysis at 100, 150, and 200°C.
3.2 In-situ Fourier transform infrared spectroscopy
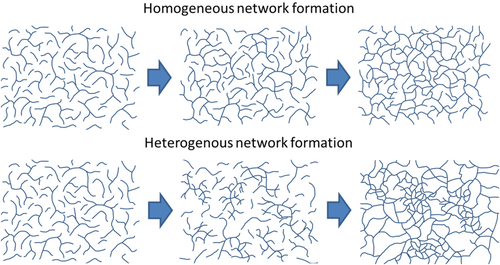
According to theories of heterogeneous network formation, during the early stages of the cure, intra-cluster cross-linking reactions (i.e., the pre-cursors to nodule structures) do not contribute towards the formation of a continuous network, Scheme 2.15 Therefore, where internal nodular morphologies are formed, the conversion at gel point should be higher.
In order to assess conversion, the epoxy-phenolic mixture was applied to a KBr disc mounted in a heated cell, and spectra were continually collected for 120 min at 100, 150, and 200°C. The cross-linking reaction was characterized by the consumption of the characteristic epoxy band at 907 cm−1, attributed to the asymmetric ether stretch of glycidyl groups, Figure 2.30, 31 This was found to occur at the same time as the growth of a band at 1108 cm−1, assigned to the asymmetric stretch of the secondary hydroxyl groups produced during the epoxy-phenolic reaction.13, 14, 32
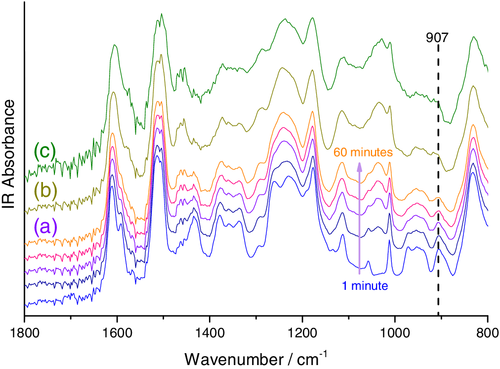
Integration of the characteristic epoxy band at 907 cm−1, and normalization to the aromatic band at 1504 cm−1, allowed epoxy consumption to be compared in each case, Figure 3a. For the higher temperature cure reactions, epoxy levels are rapidly depleted (within 10 and 1 min at 150 and 200°C, respectively) and then level off, indicating a complete reaction. In contrast, at 100°C rapid initial epoxy consumption is followed by more gradual decrease in absorbance. This is attributed to early vitrification of the resin and hence slowed diffusion of reactants, since the Tg of specimens cured for 60 min were found to lie far above the 100°C cure temperature (Tg values of 158, 174, and 187°C were recorded for resins cured for 60 min at 100°C, 15 min at 150°C and 5 min at 200°C, respectively). Integration of the 1108 cm−1 secondary hydroxyl band revealed an inversely gradual evolution of absorbance at 100°C, whilst a plateau was again rapidly established at higher cure temperatures, confirming a complete reaction in these cases, Figure 3b. It is notable that this plateau occurs at lower values of normalized absorbance for both higher temperature cures, indicating lower concentrations of secondary hydroxyl groups are present in these fully cured resins. This is consistent with previously reported activation of TGDDM homopolymerization at cure temperatures 25 ≥ 177°C.33 In addition, we have previously demonstrated that epoxy – secondary hydroxyl reactions are accelerated by the phosphonium bromide catalyst used here, the addition of which reduces the selectivity of the epoxy-phenolic reaction.14 Here, the lower final concentration of both epoxy and secondary hydroxyl groups in resins cured at 150 and 200°C indicates that reaction between the epoxy and secondary hydroxyl moieties occurs at temperatures ≥150°C.
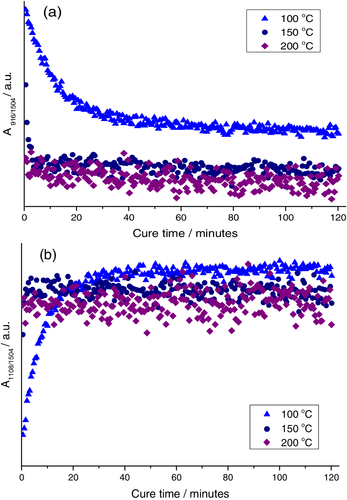
Gel points were in each case estimated using the dissolution method, and were found to occur between 1–3, 3–5, and 30–35 min at 200, 150, and 100°C, respectively. When compared with the transmission mode spectrum of freshly prepared epoxy-phenol mixture, this corresponds to epoxy consumption at the gel point of 60%–64%, 84%–85%, and 86%–92% at 100, 150, and 200°C, respectively. All of these values are significantly higher than the theoretical Flory-Stockmeyer gel point at 41% conversion for an ideal reaction, further indicating a significant contribution of intra-cluster cross-linking reactions. Both the significant jump in calculated epoxy consumption at gel point between 100 and 150°C, and the indication of increasing contributions of epoxy-hydroxyl side reactions at 150 and 200°C are consistent with extensive pre-gelation intra-domain cross-linking, and the subsequent development of distinct nodular morphologies.
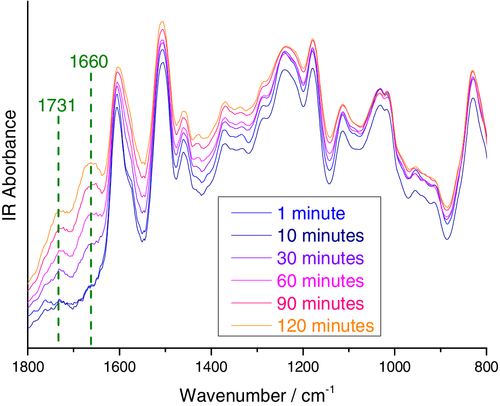
During the cure at 200°C, the emergence of characteristic epoxy oxidation bands at 1660 and 1731 cm−1 was noted, alongside broadening of the band at 1604 cm−1. These infrared bands were detected after 15 min of curing at 200°C (i.e., after epoxy consumption rates indicates the cross-linking reaction is complete), Figure 4, but were not observed to develop at any time up to 120 min of monitoring at 100 or 150°C. This infrared signature of oxidation has been widely reported to appear for epoxy-amine resins exposed to UV, hygrothermal aging, thermal oxidation conditions, and environmental weathering.34-40 Band assignments vary in the literature, but broadly, the 1731 cm−1 band corresponds to carbonyl groups, whereas absorbance around 1660 cm−1 is associated with alkene, imine, azadiene, oxime, or amide functional groups.34, 36, 37, 41-46 In the case of TGGDM cross-linked with diaminodiphenyl sulfone, dehydration and the formation of propenal has also been reported to occur at temperatures >125°C.33 Since all these functional groups are all highly polar, oxidation is expected to influence water sorption behavior. Thus, in order to minimize the contribution of oxidized material to water uptake experiments, specimens cured for 5 min at 200°C, 15 min at 150°C, and 60 min at 100°C for were selected for analysis. Despite the minor differences in the cure progression noted above, FTIR analysis indicates that the resins have comparable chemical structures at these points and epoxy consumption has leveled off, Figure 2.
3.3 Dynamic vapor sorption
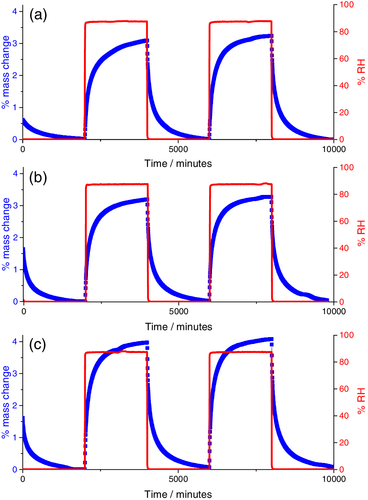
Dynamic vapor sorption (DVS) involves automated micro-gravimetric analysis under controlled humidity conditions, and thus yields precise kinetic water uptake data.47 Here, specimens were subjected cycles of 2000 min at 0% and 90% RH at 30°C, while the mass was continually monitored. Figure 5 shows the data for several mg of specimens cured for 60 min at 100°C; 15 min at 150°C, and 5 min at 200°C. For DVS experiments specimens are suspended in a glass pan housed in a temperature-controlled humidity chamber. Humidity readings and mass changes are shown for two full cycles and the initial drying step, where specimens are held at 0% RH for 2000 min to remove moisture adsorbed during storage. Percentage mass change is reported relative to the mass of the sample at the end of this initial drying step.
Comparison of the sorption isotherms for the sample cured at 150°C and the specimen cured at 100°C revealed very similar equilibrium water sorption values, Figure 6. This indicates that any difference in cure degree did not significantly impact the resin polarity, since this would be anticipated to result in increased water uptake.10, 23-28 Moreover, these results demonstrate that the development of an internal nodular nanostructure within the specimens cured at 150°C does not exert any significant influence on the equilibrium water sorption values. This is in keeping with our previous work demonstrating that for epoxy-phenolic coatings, the overall degree of cross-linking was a stronger determinant of water uptake than the internal nanostructure (distribution of cross-linking).14 It can, however, be seen that for the first water uptake cycle, the specimen displaying a nodular internal morphology (cured at 150°C) displayed slightly faster sorption kinetics. This difference was, however, eliminated on the second water sorption cycle and desorption isotherms were also found to be identical. Since any nanostructural effect on kinetics would be anticipated to persist, this increase in water sorption kinetics is instead considered symptomatic of larger degrees of unoccupied volume present in resins cured at 150°C, where plasticizing water leads to accelerated polymeric relaxation and hence densification on desorption, eliminating this difference in kinetics for the second cycle. This explanation is in keeping with literature for epoxy water sorption, however direct analysis of the unoccupied volume using, for example, positron annihilation spectroscopy would be needed for confirmation.48-50 In the present case, modulated DSC analysis provided some supporting evidence. During modulated DSC, non-reversible heat capacity traces allow the endothermic peak associated with enthalpic recovery upon heating beyond the Tg to be resolved. The magnitude of this peak represents the extent of physical aging during storage (i.e., relaxation below the Tg, associated with densification), Figure 7. Decreased enthalpy recovery as a function of cure time therefore indicates that samples cured at 100°C relaxed more readily during storage at ambient temperature, and this is associated with a decrease in the unoccupied volume.
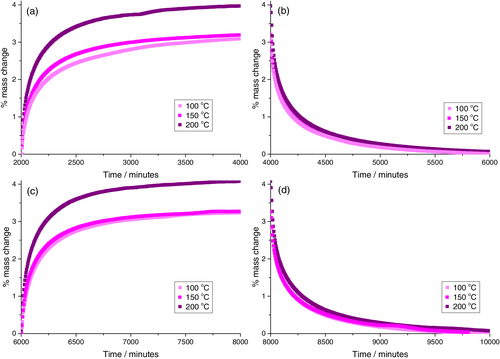
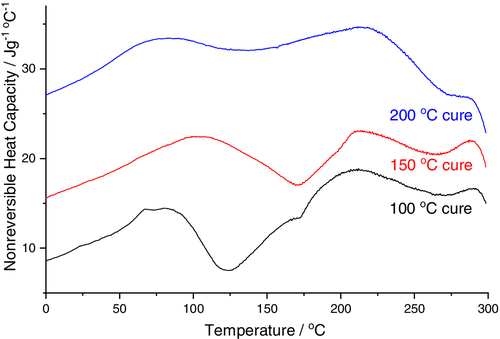
Finally, both the rate and extent of water sorption into the specimens cured at 200°C was significantly higher than those cured at 100 and 150°C, Figure 6. Numerous potential explanations for this can, however, be ruled out based on our analysis. First, AFM analysis indicates that the resins cured at 150 and 200°C resins have similar internal nanostructures. Furthermore, FTIR analysis of the cure progression indicates that the extent of reaction (epoxy consumption and secondary hydroxyl generation) is comparable for resins cured at 150 and 200°C, so a significant difference in the overall degree of cross-linking can be ruled out. In-situ FTIR analysis did, however, demonstrate that rapid oxidation is activated at 200°C, and not at 150°C. Whilst the data indicates that the extent of oxidation for resins cured for 5 min at 200°C is minimal, the oxidation of highly cross-linked resins is known to proceed via diffusion limited mechanisms, initiating at the polymer-air interface and progressing into the bulk of the material.36, 39, 45, 46, 51, 52 Since oxidation of the resin is clearly rapid at 200°C, some degree of surface oxidation is expected to be present before it is detectable by infrared analysis. We therefore attribute enhanced water sorption into the resins cured at 200°C to the introduction of polar functional groups at the surface of these coatings by oxidation. This finding is potentially significant in the case of highly cross-linked network polymers based on tetra glycidyl diaminodiphenyl methane (TGDDM), since these are commonly deployed in demanding environments, for example, aerospace, where oxidation during service is expected to be significant.
4 CONCLUSIONS
The interrelation between cure schedule and the development of heterogeneous internal morphology is successfully exploited to isolate the effect of topology on water sorption for highly cross-linked network polymers based on tetra glycidyl diaminodiphenyl methane (TGGDM) and a 1,1,1-tris(4-hydroxyphenyl)ethane cross-linker. Distinct nodular morphologies were confirmed to arise at high cure temperatures (150 and 200°C), when intra-domain cross-linking led to excess epoxy consumption prior to the gel point. In-situ FTIR data further allowed cure times to be selected where the overall cure degree and chemical functionality of resins were comparable, eliminating potentially dominant hydrogen-bonding effects on water sorption. The adsorption isotherms of heterogeneous specimens cured at 150°C in this way were obtained using dynamic vapor sorption analysis, and closely matched those of the more homogenous specimens cured at 100°C. This demonstrated that when chemical structure is controlled, the presence of a heterogeneous internal nanostructure has a negligible effect on water sorption. On the other hand, a minimal degree of surface oxidation leads to a significant increase in equilibrium water uptake for the specimens cured at 200°C, when compared to those prepared at 150°C. This finding is significant since oxidation is well-known to occur during the service lifetime of many adhesives, coatings and composites based on epoxy resins, even under mild conditions.
AUTHOR CONTRIBUTIONS
Suzanne Morsch: Conceptualization (lead); data curation (lead); formal analysis (lead); investigation (lead); methodology (lead); writing – original draft (lead). Stuart B. Lyon: Funding acquisition (lead); project administration (equal); supervision (lead); writing – review and editing (equal). Simon R. Gibbon: Conceptualization (supporting); funding acquisition (equal); supervision (supporting); writing – review and editing (equal). Mark Irwin: Funding acquisition (supporting); project administration (equal); supervision (supporting); writing – review and editing (equal).
ACKNOWLEDGMENTS
S. Morsch is grateful to the EPSRC (grant number EP/S004963/1) and AkzoNobel for materials and financial support.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.