Self-Activated Heterogeneous Fenton Process for Accelerated Degradation of Aromatic Pollutants over Copper Oxide Catalysts
Graphical Abstract
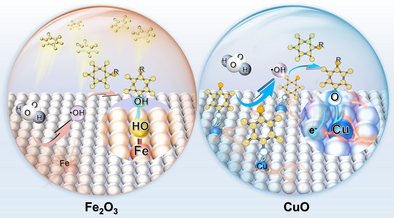
This study reports an unusual phenomenon of pollutant-induced activity enhancement of CuO for H2O2 activation and self-accelerated pollutant degradation, contrasting the performance decay of Fe2O3 catalyst. CuO can stabilize and activate phenol via ligand-to-metal charge transfer, generating surface-bound phenoxyl radicals to further mediate the H2O2 activation and meanwhile yielding low-valent Cu. This offers opportunities for simultaneously addressing the catalyst passivation and redox cycling limitations of heterogeneous Fenton-like catalysis.
Abstract
Metal-based heterogeneous catalysts have been commonly adopted for Fenton-like oxidation of organic pollutants, but generally suffer from inadequate activity in practical water treatment applications due to surface passivation by accumulated pollutants and sluggish redox cycling of active metal. Here, we observed an unusual phenomenon of pollutant-induced activity enhancement for copper oxide (CuO) in H2O2 activation and phenol degradation, which is in sharp contrast to considerable activity decay of Fe2O3 catalyst. The CuO was found to stabilize and activate phenol via ligand-to-metal charge transfer route, generating surface-bound phenoxyl radicals for further mediating the H2O2 activation and enabling a rapid regeneration of low-valent Cu. Based on this principle, a Fe-Cu bimetal oxides catalyst was elaborated to further augment the catalyst-phenol interaction towards self-activated Fenton oxidation. The optimal catalyst achieved 14-time faster pollutant degradation rate and 2 order-of-magnitude higher H2O2 utilization efficiency than the Fe2O3 control. It also demonstrated good adaptability to degradation of diverse substituted benzenes and maintained stable performance for treatment of real lake water during 100-day continuous operation. Our work implies that the catalyst-pollutant interaction may be rationally leveraged and modulated to create highly efficient and stable heterogeneous catalytic systems, thus further unlocking their potential for sustainable water purification application.
Conflict of Interests
The authors declare that none of the work reported in this study could have been influenced by any known competing financial interests or personal relationships.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.