Valence Tautomerism and Alkaline Properties of Global Aromatic Imine- and Methine-Bridged all-Benzenoid Macrocyclic Superpyrazine and Supertriazine
Graphical Abstract
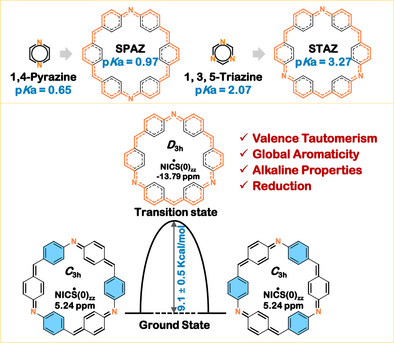
We prepared two imine macrocycles, SPAZ and STAZ, as analogues of 1,4-pyrazine and 1,3,5-triazine, respectively. SPAZ and STAZ exhibit Brønsted basicity with pKa values of 0.97 and 3.27, respectively. Notably, we elucidate their unprecedented valence tautomerism between “super-Kekulé” benzenoid/quinoidal structures and a “super-sextet” transient structure, facilitated by aza[30]annulene pathways.
Abstract
The comprehensive understanding of the ground-state electronic structures of all-benzenoid macrocycles and their charged species, particularly those exhibiting global (anti)aromaticity, remains poorly resolved. Here, we present two imine- and methine-bridged all-benzenoid [6]cyclo-para-phenylenes SPAZ (superpyrazine) and STAZ (supertriazine)-designed as π-extended analogues of 1,4-pyrazine and 1,3,5-triazine, respectively. For the first time, we elucidate their unprecedented valence tautomerism between “super-Kekulé” alternating benzenoid/quinoidal structures and a “super-sextet” transient structure, mediated by a 30π azaannulene conjugated pathway. The aromatic stabilization energy of global aromatic delocalization would partially counterbalance the energy penalty for disrupting three benzenoid sextets. Further protonation studies demonstrate their alkaline characteristics (pKa = 0.97 for SPAZ; 3.27 for STAZ), with diprotonated SPAZ-DH and monoprotonated STAZ-MH adopting planarized geometries that amplify global aromaticity through charge-induced structural constraints and enhanced π-electron delocalization. Notably, two-electron reduction generates a dianion SPAZ-DA exhibiting 32π electron global antiaromaticity with amine N atom embedded aza[30]annulene pathway. These findings establish a framework for decoding electronic structure property relationships in all-benzenoid macrocycles, offering insights into aromaticity modulated regulations through tautomerism, protonation, and redox switching.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.