Bis(pinacolato)Diboron-Enabled Nickel-Catalyzed Regio- and Enantioselective Reductive [3 + 2] Annulation of β-Bromoenones with Alkynes
Wangyang Li
Key Laboratory of Molecule Synthesis and Function Discovery, Fujian Province University, College of Chemistry at Fuzhou University, Fuzhou, Fujian, 350108 P.R.China
Search for more papers by this authorYanping Zheng
Key Laboratory of Molecule Synthesis and Function Discovery, Fujian Province University, College of Chemistry at Fuzhou University, Fuzhou, Fujian, 350108 P.R.China
Search for more papers by this authorYunya Gu
Key Laboratory of Molecule Synthesis and Function Discovery, Fujian Province University, College of Chemistry at Fuzhou University, Fuzhou, Fujian, 350108 P.R.China
Search for more papers by this authorShanshan Cheng
Key Laboratory of Molecule Synthesis and Function Discovery, Fujian Province University, College of Chemistry at Fuzhou University, Fuzhou, Fujian, 350108 P.R.China
Search for more papers by this authorJinhui Xie
Key Laboratory of Molecule Synthesis and Function Discovery, Fujian Province University, College of Chemistry at Fuzhou University, Fuzhou, Fujian, 350108 P.R.China
Search for more papers by this authorYong Lu
Key Laboratory of Molecule Synthesis and Function Discovery, Fujian Province University, College of Chemistry at Fuzhou University, Fuzhou, Fujian, 350108 P.R.China
Search for more papers by this authorShanglin Chen
Key Laboratory of Molecule Synthesis and Function Discovery, Fujian Province University, College of Chemistry at Fuzhou University, Fuzhou, Fujian, 350108 P.R.China
Search for more papers by this authorCorresponding Author
Qiuling Song
Key Laboratory of Molecule Synthesis and Function Discovery, Fujian Province University, College of Chemistry at Fuzhou University, Fuzhou, Fujian, 350108 P.R.China
State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210093 P.R.China
E-mail: [email protected]
Search for more papers by this authorWangyang Li
Key Laboratory of Molecule Synthesis and Function Discovery, Fujian Province University, College of Chemistry at Fuzhou University, Fuzhou, Fujian, 350108 P.R.China
Search for more papers by this authorYanping Zheng
Key Laboratory of Molecule Synthesis and Function Discovery, Fujian Province University, College of Chemistry at Fuzhou University, Fuzhou, Fujian, 350108 P.R.China
Search for more papers by this authorYunya Gu
Key Laboratory of Molecule Synthesis and Function Discovery, Fujian Province University, College of Chemistry at Fuzhou University, Fuzhou, Fujian, 350108 P.R.China
Search for more papers by this authorShanshan Cheng
Key Laboratory of Molecule Synthesis and Function Discovery, Fujian Province University, College of Chemistry at Fuzhou University, Fuzhou, Fujian, 350108 P.R.China
Search for more papers by this authorJinhui Xie
Key Laboratory of Molecule Synthesis and Function Discovery, Fujian Province University, College of Chemistry at Fuzhou University, Fuzhou, Fujian, 350108 P.R.China
Search for more papers by this authorYong Lu
Key Laboratory of Molecule Synthesis and Function Discovery, Fujian Province University, College of Chemistry at Fuzhou University, Fuzhou, Fujian, 350108 P.R.China
Search for more papers by this authorShanglin Chen
Key Laboratory of Molecule Synthesis and Function Discovery, Fujian Province University, College of Chemistry at Fuzhou University, Fuzhou, Fujian, 350108 P.R.China
Search for more papers by this authorCorresponding Author
Qiuling Song
Key Laboratory of Molecule Synthesis and Function Discovery, Fujian Province University, College of Chemistry at Fuzhou University, Fuzhou, Fujian, 350108 P.R.China
State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210093 P.R.China
E-mail: [email protected]
Search for more papers by this authorGraphical Abstract
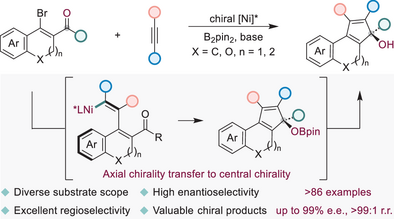
We report the first bis(pinacolato)diboron-enabled Ni-catalyzed regio- and enantioselective reductive [3 + 2] annulation of β-bromoenones with alkynes, providing convenient access to synthetically valuable chiral five-membered cyclic tertiary alcohols via axial chirality transfer to central chirality. A broad substrate scope, late-stage functionalization of complex molecules, and diverse transformations highlight the utility of this reaction.
Abstract
Chiral five-membered cyclic tertiary alcohols are important structural motifs in functional materials, pharmaceuticals, and bioactive molecules. Hence, developing efficient methodologies for synthesizing compounds featuring these privileged scaffolds represents a crucial pursuit within synthetic chemistry. Herein, we present a regio- and enantioselective Ni-catalyzed strategy for the reductive [3 + 2] annulation of β-bromoenones with alkynes, providing convenient access to chiral five-membered cyclic tertiary alcohols with high levels of regio-, and enantioselectivity via axial chirality transfer to central chirality. The utilization of an environmentally sustainable bis(pinacolato)diboron (B2pin2) is crucial for the success of this asymmetric reductive cyclization reaction. Simultaneously, the mild reaction environment greatly enhances functional group compatibility. This has been demonstrated by the broad substrate scope, late-stage functionalizations of bioactive compounds or drug molecules, and subsequent transformations. Amongst, it is worth emphasizing that these functionally enriched chiral five-membered cyclic tertiary alcohols can efficiently participate in Diels–Alder reactions to synthesize enantioenriched polycyclic and heterocyclic molecules, thereby further validating the significance of introducing a cyclopentadiene skeleton. The preliminary mechanistic studies revealed the mode of action of B2pin2 in mononuclear Ni-catalyzed asymmetric reductive [3 + 2] annulation reactions and density functional theory (DFT) calculations clarified the origin of the experimentally observed regio- and enantioselectivity.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
Supporting Information
| Filename | Description |
|---|---|
| anie202506873-sup-0001-SuppMat.pdf33.3 MB | Supporting Information |
| anie202506873-sup-0002-SuppMat.cif826.5 KB | Supporting Information |
| anie202506873-sup-0003-SuppMat.cif976.6 KB | Supporting Information |
| anie202506873-sup-0004-SuppMat.cif910.3 KB | Supporting Information |
| anie202506873-sup-0005-SuppMat.cif1.3 MB | Supporting Information |
| anie202506873-sup-0006-SuppMat.cif631.3 KB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1L. Munoz, Nat. Rev. Drug Discovery 2017, 16, 424–440.
- 2K.-Y. Lu, L.-C. Cheng, Z.-C. Hung, Z.-Y. Chen, C.-W. Wang, H.-H. Hou, in Curr. Issues Mol. Biol. 46, 2024, 2701–2712.
- 3A. Ridichie, A. Ledeţi, L. Sbârcea, G. Rusu, C. Muntean, D. Cîrcioban, F. Peter, I. Ledeţi, J. Therm. Anal. Calorim. 2024, https://doi.org/10.1007/s10973-024-13149-w.
- 4T. Tokunaga, W. E. Hume, T. Umezome, K. Okazaki, Y. Ueki, K. Kumagai, S. Hourai, J. Nagamine, H. Seki, M. Taiji, H. Noguchi, R. Nagata, J. Med. Chem. 2001, 44, 4641–4649.
- 5J. L. Stymiest, V. Bagutski, R. M. French, V. K. Aggarwal, Nature 2008, 456, 778–782.
- 6R. A. Watile, A. Bunrit, J. Margalef, S. Akkarasamiyo, R. Ayub, E. Lagerspets, S. Biswas, T. Repo, J. S. M. Samec, Nat. Commun. 2019, 10, 3826.
- 7M. Tang, H. Gu, S. He, S. Rajkumar, X. Yang, Angew. Chem. Int. Ed. 2021, 60, 21334–21339.
- 8S. Rajkumar, M. Tang, X. Yang, Angew. Chem. Int. Ed. 2020, 59, 2333–2337.
- 9T.-Y. Zhao, L.-J. Xiao, Q.-L. Zhou, Angew. Chem. Int. Ed. 2022, 61, e202115702.
- 10B.-B. Gou, W.-J. Shen, Y.-J. Gao, Q. Gu, S.-L. You, Sci. China Chem. 2025, 68, https://doi.org/10.1007/s11426-024-2514-1.
- 11B. Ding, Q. Xue, S. Jia, H.-G. Cheng, Q. Zhou, Synthesis 2022, 54, 1721–1732.
- 12A. V. R. Madduri, S. R. Harutyunyan, A. J. Minnaard, Drug Discov. Today Technol. 2013, 10, e21–e27.
- 13M. Shibasaki, M. Kanai, Chem. Rev. 2008, 108, 2853–2873.
- 14C. Garcia, S. V. Martin, Curr. Org. Chem. 2006, 10, 1849–1889.
- 15Y.-L. Liu, X.-T. Lin, Adv. Synth. Catal. 2019, 361, 876–918.
- 16L. Hou, X. Liu, W. Cao, X. Feng, ChemCatChem 2023, 15, e202300893.
- 17J. Rong, T. Pellegrini, S. R. Harutyunyan, Chem. - Eur. J. 2016, 22, 3558–3570.
- 18L. Pu, H.-B. Yu, Chem. Rev. 2001, 101, 757–824.
- 19P. Knochel, W. Dohle, N. Gommermann, F. F. Kneisel, F. Kopp, T. Korn, I. Sapountzis, V. A. Vu, Angew. Chem. Int. Ed. 2003, 42, 4302–4320.
- 20J.-Q. Chen, Z.-B. Dong, Synthesis 2020, 52, 3714–3734.
- 21C. Clarke, C. A. Incerti-Pradillos, H. W. Lam, J. Am. Chem. Soc. 2016, 138, 8068–8071.
- 22H. Green, S. P. Argent, H. W. Lam, Chem. - Eur. J. 2021, 27, 5897–5900.
- 23B. Sun, L.-X. Ruan, R. Zhao, J. Zhang, R. Niu, Q. Luo, Y. Zhang, L. Gao, S.-L. Shi, Nat. Synth. 2024, 3, 1091–1103.
- 24Y. Cai, L.-X. Ruan, A. Rahman, S.-L. Shi, Angew. Chem. Int. Ed. 2021, 60, 5262–5267.
- 25L.-X. Ruan, B. Sun, J.-M. Liu, S.-L. Shi, Science 2023, 379, 662–670.
- 26R. Shintani, M. Inoue, T. Hayashi, Angew. Chem. Int. Ed. 2006, 45, 3353–3356.
- 27G. Liu, X. Lu, J. Am. Chem. Soc. 2006, 128, 16504–16505.
- 28H.-F. Duan, J.-H. Xie, X.-C. Qiao, L.-X. Wang, Q.-L. Zhou, Angew. Chem. Int. Ed. 2008, 47, 4351–4353.
- 29F. Cai, X. Pu, X. Qi, V. Lynch, A. Radha, J. M. Ready, J. Am. Chem. Soc. 2011, 133, 18066–18069.
- 30T.-S. Zhu, S.-S. Jin, M.-H. Xu, Angew. Chem. Int. Ed. 2012, 51, 780–783.
- 31L. Huang, J. Zhu, G. Jiao, Z. Wang, X. Yu, W.-P. Deng, W. Tang, Angew. Chem. Int. Ed. 2016, 55, 4527–4531.
- 32Y. Huang, R.-Z. Huang, Y. Zhao, J. Am. Chem. Soc. 2016, 138, 6571–6576.
- 33H. Wei, Y. Luo, J. Ren, Q. Yuan, W. Zhang, Nat. Commun. 2024, 15, 8775.
- 34K. E. Poremba, S. E. Dibrell, S. E. Reisman, ACS Catal. 2020, 10, 8237–8246.
- 35Q. Pan, Y. Ping, W. Kong, Acc. Chem. Res. 2023, 56, 515–535.
- 36L. J. Oxtoby, J. A. Gurak, S. R. Wisniewski, M. D. Eastgate, K. M. Engle, Trends Chem 2019, 1, 572–587.
- 37W. Xu, T. Xu, Acc. Chem. Res. 2024, 57, 1997–2011.
- 38L.-M. Chen, S. E. Reisman, Acc. Chem. Res. 2024, 57, 751–762.
- 39T. Moragas, A. Correa, R. Martin, Chem. - Eur. J. 2014, 20, 8242–8258.
- 40S. Huang, J. S. Zhou, J. Am. Chem. Soc. 2024, 146, 12895–12900.
- 41X. Jiang, H. Jiang, Q. Yang, Y. Cheng, L.-Q. Lu, J. A. Tunge, W.-J. Xiao, J. Am. Chem. Soc. 2022, 144, 8347–8354.
- 42K. Yabushita, A. Yuasa, K. Nagao, H. Ohmiya, J. Am. Chem. Soc. 2019, 141, 113–117.
- 43H. Jiang, X.-K. He, X. Jiang, W. Zhao, L.-Q. Lu, Y. Cheng, W.-J. Xiao, J. Am. Chem. Soc. 2023, 145, 6944–6952.
- 44Z. Zhu, J. Xiao, M. Li, Z. Shi, Angew. Chem. Int. Ed. 2022, 61, e202201370.
- 45S. Zhang, S. Perveen, Y. Ouyang, L. Xu, T. Yu, M. Zhao, L. Wang, P. Song, P. Li, Angew. Chem. Int. Ed. 2022, 61, e202117843.
- 46B. Sun, Z.-H. Wang, Y.-Z. Wang, Y.-C. Gu, C. Ma, T.-S. Mei, Sci. Bull. 2023, 68, 2033–2041.
- 47P. Zhou, T. Xu, Chem. Commun. 2020, 56, 8194–8197.
- 48Y. Li, W. Li, J. Tian, G. Huang, H. Lv, Org. Lett. 2020, 22, 5353–5357.
- 49Y.-F. Han, Y. Li, X.-H. Ouyang, M. Hu, Z. Tan, J.-H. Li, ACS Catal. 2021, 11, 10115–10122.
- 50H. Xia, X. Jiang, D. Lin, S. Zhang, Z. Yu, X. Wu, J. Qu, Y. Chen, J. Am. Chem. Soc. 2024, 146, 28468–28481.
- 51D. J. Charboneau, H. Huang, E. L. Barth, C. C. Germe, N. Hazari, B. Q. Mercado, M. R. Uehling, S. L. Zultanski, J. Am. Chem. Soc. 2021, 143, 21024–21036.
- 52M. C. Franke, V. R. Longley, M. Rafiee, S. S. Stahl, E. C. Hansen, D. J. Weix, ACS Catal. 2022, 12, 12617–12626.
- 53Z.-M. Su, R. Deng, S. S. Stahl, Nat. Chem. 2024, 16, 2036–2043.
- 54D. Sun, Y. Gong, Y. Wu, Y. Chen, H. Gong, Adv. Sci. 2024, 11, 2404301.
- 55T. Ishiyama, N. Miyaura, Chem. Rec. 2004, 3, 271–280.
- 56J. Hu, M. Ferger, Z. Shi, T. B. Marder, Chem. Soc. Rev. 2021, 50, 13129–13188.
- 57X. Ma, Z. Kuang, Q. Song, JACS Au 2022, 2, 261–279.
- 58A. Whyte, A. Torelli, B. Mirabi, A. Zhang, M. Lautens, ACS Catal. 2020, 10, 11578–11622.
- 59Z. Fan, M. Ye, Y. Wang, J. Qiu, W. Li, X. Ma, K. Yang, Q. Song, ACS Cent. Sci. 2022, 8, 1134–1144.
- 60J. Xie, W. Li, Y. Lu, Y. Zheng, Y. Huang, S. Chen, Q. Song, J. Am. Chem. Soc. 2024, 146, 10167–10176.
- 61W. Li, H. Chen, Y. Zheng, Y. Lu, J. Xie, S. Chen, Y. Lan, Q. Song, ACS Catal. 2024, 14, 11318–11331.
- 62M. Ke, Q. Feng, K. Yang, Q. Song, Org. Chem. Front. 2016, 3, 150–155.
- 63Q. Xuan, C. Zhao, Q. Song, Org. Biomol. Chem. 2017, 15, 5140–5144.
- 64Q. Xuan, W. Kong, Q. Song, J. Org. Chem. 2017, 82, 7602–7607.
- 65F. Ke, C. Yu, X. Li, H. Sheng, Q. Song, Org. Lett. 2023, 25, 2733–2738.
- 66S. Geng, C. Shi, B. Guo, H. Hou, Z. Liu, Z. Feng, ACS Catal. 2023, 13, 15469–15480.
- 67Z. Zhu, L. Lin, J. Xiao, Z. Shi, Angew. Chem. Int. Ed. 2022, 61, e202113209.
- 68K. Yang, Q. Song, Acc. Chem. Res. 2021, 54, 2298–2312.
- 69Z. Kuang, K. Yang, Y. Zhou, Q. Song, Chem. Commun. 2020, 56, 6469–6479.
- 70W. Li, S. Chen, J. Xie, Z. Fan, K. Yang, Q. Song, Nat. Synth. 2023, 2, 140–151.
- 71Z.-S. Liu, Y. Hua, Q. Gao, Y. Ma, H. Tang, Y. Shang, H.-G. Cheng, Q. Zhou, Nat. Catal. 2020, 3, 727–733.
- 72Deposition Numbers https://www.ccdc.cam.ac.uk/services/structures?id= https://doi.org/10.1002/anie.202506873 %2387805 (for 3), 2387806 (for 95), 2387807 (for 96), 2387793 (for 99), 2387788 (for 101) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe href = http://www.ccdc.cam.ac.uk/structures Access Structures service.
10.1002/anie.202506873 Google Scholar
- 73S. J. Miller, T. Ritter, Chem. Rev. 2023, 123, 13867–13868.
- 74A. L. Lane, S.-J. Nam, T. Fukuda, K. Yamanaka, C. A. Kauffman, P. R. Jensen, W. Fenical, B. S. Moore, J. Am. Chem. Soc. 2013, 135, 4171–4174.
- 75K. Yamada, M. J. Lear, T. Yamaguchi, S. Yamashita, I. D. Gridnev, Y. Hayashi, M. Hirama, Angew. Chem. Int. Ed. 2014, 53, 13902–13906.
- 76J. Wang, G. Dong, Chem. Rev. 2019, 119, 7478–7528.
- 77H.-G. Cheng, S. Jia, Q. Zhou, Acc. Chem. Res. 2023, 56, 573–591.
- 78C.-X. Gu, W.-W. Chen, M.-H. Xu, J. Org. Chem. 2020, 85, 3887–3893.
- 79M. Yang, X. Zhang, X. Lu, Org. Lett. 2007, 9, 5131–5133.
- 80P. Zheng, W. Xu, H. Wang, D. Wang, X. Wu, T. Xu, ACS Catal. 2022, 12, 14926–14933.
- 81K. Li, X. Long, S. Zhu, ACS Catal. 2023, 13, 2422–2431.
- 82T. Lu, F. Chen, J. Comput. Chem. 2012, 33, 580–592.