Copper-Photoredox-Catalyzed C(sp3)–C(sp3) Reductive Cross-Coupling of Alkyl Bromides with BCP-Thianthrenium Reagents
Saikat Pandit
Max-Planck-Institut für Kohlenforschung, Mülheim an der Ruhr, Germany
Institute of Organic Chemistry, RWTH Aachen University, Aachen, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Tobias Ritter
Max-Planck-Institut für Kohlenforschung, Mülheim an der Ruhr, Germany
Institute of Organic Chemistry, RWTH Aachen University, Aachen, Germany
E-mail: [email protected]
Search for more papers by this authorSaikat Pandit
Max-Planck-Institut für Kohlenforschung, Mülheim an der Ruhr, Germany
Institute of Organic Chemistry, RWTH Aachen University, Aachen, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Tobias Ritter
Max-Planck-Institut für Kohlenforschung, Mülheim an der Ruhr, Germany
Institute of Organic Chemistry, RWTH Aachen University, Aachen, Germany
E-mail: [email protected]
Search for more papers by this authorGraphical Abstract
Abstract
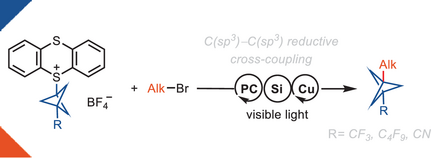
Herein, we report a reductive cross-coupling reaction of bicyclo[1.1.1]pentyl (BCP)-thianthrenium reagents and alkyl bromides. The reaction is catalyzed by a copper/photoredox catalyst system. The approach is the first example of a cross-coupling between BCP-based reagents with alkyl electrophiles.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supporting information of this article.
Supporting Information
| Filename | Description |
|---|---|
| anie202506785-sup-0001-SuppMat.pdf6.4 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1A. F. Stepan, C. Subramanyam, I. V. Efremov, J. K. Dutra, T. J. O'Sullivan, K. J. DiRico, W. S. McDonald, A. Won, P. H. Dorff, C. E. Nolan, S. L. Becker, L. R. Pustilnik, D. R. Riddell, G. W. Kauffman, B. L. Kormos, L. Zhang, Y. Lu, S. H. Capetta, M. E. Green, K. Karki, E. Sibley, K. P. Atchison, A. J. Hallgren, C. E. Oborski, A. E. Robshaw, B. Sneed, C. J. O'Donnell, J. Med. Chem. 2012, 55, 3414–3424.
- 2Y. P. Auberson, C. Brocklehurst, M. Furegati, T. C. Fessard, G. Koch, A. Decker, L. La Vecchia, E. Briard, ChemMedChem 2017, 12, 590–598.
- 3Y. L. Goh, Y. T. Cui, V. Pendharkar, V. A. Adsool, ACS Med. Chem. Lett. 2017, 8, 516–520.
- 4N. D. Measom, K. D. Down, D. J. Hirst, C. Jamieson, E. S. Manas, V. K. Patel, D. O. Somers, ACS Med. Chem. Lett. 2017, 8, 43–48.
- 5A. Nilova, L.-C. Campeau, E. C. Sherer, D. R. Stuart, J. Med. Chem. 2020, 63, 13389–13396.
- 6M. A. M. Subbaiah, N. A. Meanwell, J. Med. Chem. 2021, 64, 14046–14128.
- 7D. F. J. Caputo, C. Arroniz, A. B. Dürr, J. J. Mousseau, A. F. Stepan, S. J. Mansfield, E. A. Anderson, Chem. Sci. 2018, 9, 5295–5300.
- 8M. Kondo, J. Kanazawa, T. Ichikawa, T. Shimokawa, Y. Nagashima, K. Miyamoto, M. Uchiyama, Angew. Chem. Int. Ed. 2020, 59, 1970–1974.
- 9I. S. Makarov, C. E. Brocklehurst, K. Karaghiosoff, G. Koch, P. Knochel, Angew. Chem. Int. Ed. 2017, 56, 12774–12777.
- 10J. Nugent, B. R. Shire, D. F. J. Caputo, H. D. Pickford, F. Nightingale, I. T. T. Houlsby, J. J. Mousseau, E. A. Anderson, Angew. Chem. Int. Ed. 2020, 59, 11866–11870.
- 11W. Dong, E. Yen-Pon, L. Li, A. Bhattacharjee, A. Jolit, G. A. Molander, Nat. Chem. 2022, 14, 1068–1077.
- 12I. F. Yu, J. L. Manske, A. Dieguez-Vazquez, A. Misale, A. E. Pashenko, P. K. Mykhailiuk, S. V. Ryabukhin, D. M. Volochnyuk, J. F. Hartwig, Nat. Chem. 2023, 15, 685–693.
- 13M. D. VanHeyst, J. Qi, A. J. Roecker, J. M. E. Hughes, L. Cheng, Z. Zhao, J. Yin, Org. Lett. 2020, 22, 1648–1654.
- 14F. Toriyama, J. Cornella, L. Wimmer, T. G. Chen, D. D. Dixon, G. Creech, P. S. Baran, J. Am. Chem. Soc. 2016, 138, 11132–11135.
- 15V. C. Polites, S. O. Badir, S. Keess, A. Jolit, G. A. Molander, Org. Lett. 2021, 23, 4828–4833.
- 16S. K. V. Vernekar, Z. Liu, E. Nagy, L. Miller, K. A. Kirby, D. J. Wilson, J. Kankanala, S. G. Sarafianos, M. A. Parniak, Z. Wang, J. Med. Chem. 2014, 58, 651–664.
- 17S. Mondal, G. Panda, RSC Adv. 2014, 4, 28317–28358.
- 18C. Manzoni, M. R. Lovati, A. Bonelli, G. Galli, C. R. Sirtori, Eur. J. Pharmacol. 1990, 190, 39–49.
- 19M. Valipour, H. Irannejad, S. Emami, Drug Dev. Res. 2022, 83, 1246–1250.
- 20F. R. Walter, S. Veszelka, M. Pásztói, Z. A. Péterfi, A. Tóth, G. Rákhely, L. Cervenak, C. S. Ábrahám, M. A. Deli, J. Neurochem. 2015, 134, 1040–1054.
- 21E. M. Alvarez, Z. Bai, S. Pandit, N. Frank, L. Torkowski, T. Ritter, Nat. Synth. 2023, 2, 548–556.
- 22Z. Bai, B. Lansbergen, T. Ritter, J. Am. Chem. Soc. 2023, 145, 25954–25961.
- 23J. Nugent, C. Arroniz, B. R. Shire, A. J. Sterling, H. D. Pickford, M. L. J. Wong, S. J. Mansfield, D. F. J. Caputo, B. Owen, J. J. Mousseau, F. Duarte, E. A. Anderson, ACS Catal. 2019, 9, 9568–9574.
- 24L.-C. Campeau, N. Hazari, Organometallics 2018, 38, 3–35.
- 25J. Magano, J. R. Dunetz, Chem. Rev. 2011, 111, 2177–2250.
- 26D. A. Everson, D. J. Weix, J. Org. Chem. 2014, 79, 4793–4798.
- 27R. Kranthikumar, Organometallics 2022, 41, 667–679.
- 28F. Lovering, J. Bikker, C. Humblet, J. Med. Chem. 2009, 52, 6752–6756.
- 29Y. Hioki, M. Costantini, J. Griffin, K. C. Harper, M. P. Merini, B. Nissl, Y. Kawamata, P. S. Baran, Science 2023, 380, 81–87.
- 30C. M. Seong, A. Q. Ansel, C. C. Roberts, J. Org. Chem. 2023, 88, 3935–3940.
- 31A. B. Sanford, T. A. Thane, T. M. McGinnis, P.-P. Chen, X. Hong, E. R. Jarvo, J. Am. Chem. Soc. 2020, 142, 5017–5023.
- 32J. B. Diccianni, J. Katigbak, C. Hu, T. Diao, J. Am. Chem. Soc. 2019, 141, 1788–1796.
- 33X. F. Yu, T. Yang, S. Wang, H. Xu, H. Gong, Org. Lett. 2011, 13, 2138–2141.
- 34H. Xu, C. Zhao, Q. Qian, W. Deng, H. Gong, Chem. Sci. 2013, 4, 4022–4022.
- 35Z. Liang, W. Xue, K. Lin, H. Gong, Org. Lett. 2014, 16, 5620–5623.
- 36J. H. Liu, C. T. Yang, X. Y. Lu, Z. Q. Zhang, L. Xu, M. Cui, X. Lu, B. Xiao, Y. Fu, L. Liu, Chem. - Eur. J. 2014, 20, 15334–15338.
- 37R. T. Smith, X. Zhang, J. A. Rincón, J. Agejas, C. Mateos, M. Barberis, S. García-Cerrada, O. de Frutos, D. W. C. MacMillan, J. Am. Chem. Soc. 2018, 140, 17433–17438.
- 38T. Yang, Y. Wei, M. J. Koh, ACS Catal. 2021, 11, 6519–6525.
- 39K. Kang, D. J. Weix, Org. Lett. 2022, 24, 2853–2857.
- 40M. Y. S. Ibrahim, G. R. Cumming, R. G. de Vega, P. Garcia-Losada, O. de Frutos, C. O. Kappe, D. Cantillo, J. Am. Chem. Soc. 2023, 145, 17023–17028.
- 41Y. Li, Y. Li, L. Peng, D. Wu, L. Zhu, G. Yin, Chem. Sci. 2020, 11, 10461–10464.
- 42A. H. Cherney, S. E. Reisman, J. Am. Chem. Soc. 2014, 136, 14365–14368.
- 43W. Zhang, L. Lu, W. Zhang, Y. Wang, S. D. Ware, J. Mondragon, J. Rein, N. Strotman, D. Lehnherr, K. A. See, S. Lin, Nature 2022, 604, 292–297.
- 44M. J. Gibian, R. C. Corley, Chem. Rev. 1973, 73, 441–464.
- 45D. J. P. Kornfilt, D. W. C. MacMillan, J. Am. Chem. Soc. 2019, 141, 6853–6858.
- 46C. Le, T. Q. Chen, T. Liang, P. Zhang, D. W. C. MacMillan, Science 2018, 360, 1010–1014.
- 47W. Dong, S. Keess, G. A. Molander, Chem Catalysis 2023, 3, 100608.