Enantioselective Decarboxylative Acylation of α-Hydroxy Acids with Carboxylic Acids via Photoredox/Nickel Dual Catalysis
Chen-Qiang Deng
State Key Laboratory of Precision and Intelligent Chemistry, CAS Key Laboratory of Urban Pollutant Conversion, Anhui Province Key Laboratory of Biomass Chemistry, Department of Applied Chemistry, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorPeng-Pai Liu
State Key Laboratory of Precision and Intelligent Chemistry, CAS Key Laboratory of Urban Pollutant Conversion, Anhui Province Key Laboratory of Biomass Chemistry, Department of Applied Chemistry, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorYuantai Xu
State Key Laboratory of Precision and Intelligent Chemistry, CAS Key Laboratory of Urban Pollutant Conversion, Anhui Province Key Laboratory of Biomass Chemistry, Department of Applied Chemistry, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorXue-Bin Zhang
State Key Laboratory of Precision and Intelligent Chemistry, CAS Key Laboratory of Urban Pollutant Conversion, Anhui Province Key Laboratory of Biomass Chemistry, Department of Applied Chemistry, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Jin Deng
State Key Laboratory of Precision and Intelligent Chemistry, CAS Key Laboratory of Urban Pollutant Conversion, Anhui Province Key Laboratory of Biomass Chemistry, Department of Applied Chemistry, University of Science and Technology of China, Hefei, 230026 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Prof. Dr. Yao Fu
State Key Laboratory of Precision and Intelligent Chemistry, CAS Key Laboratory of Urban Pollutant Conversion, Anhui Province Key Laboratory of Biomass Chemistry, Department of Applied Chemistry, University of Science and Technology of China, Hefei, 230026 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorChen-Qiang Deng
State Key Laboratory of Precision and Intelligent Chemistry, CAS Key Laboratory of Urban Pollutant Conversion, Anhui Province Key Laboratory of Biomass Chemistry, Department of Applied Chemistry, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorPeng-Pai Liu
State Key Laboratory of Precision and Intelligent Chemistry, CAS Key Laboratory of Urban Pollutant Conversion, Anhui Province Key Laboratory of Biomass Chemistry, Department of Applied Chemistry, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorYuantai Xu
State Key Laboratory of Precision and Intelligent Chemistry, CAS Key Laboratory of Urban Pollutant Conversion, Anhui Province Key Laboratory of Biomass Chemistry, Department of Applied Chemistry, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorXue-Bin Zhang
State Key Laboratory of Precision and Intelligent Chemistry, CAS Key Laboratory of Urban Pollutant Conversion, Anhui Province Key Laboratory of Biomass Chemistry, Department of Applied Chemistry, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Jin Deng
State Key Laboratory of Precision and Intelligent Chemistry, CAS Key Laboratory of Urban Pollutant Conversion, Anhui Province Key Laboratory of Biomass Chemistry, Department of Applied Chemistry, University of Science and Technology of China, Hefei, 230026 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Prof. Dr. Yao Fu
State Key Laboratory of Precision and Intelligent Chemistry, CAS Key Laboratory of Urban Pollutant Conversion, Anhui Province Key Laboratory of Biomass Chemistry, Department of Applied Chemistry, University of Science and Technology of China, Hefei, 230026 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorGraphical Abstract
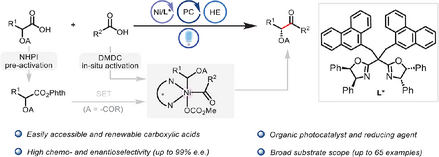
A photoredox/nickel dual-catalyzed enantioselective decarboxylative acylation of α-hydroxy acid derivatives with carboxylic acids has been developed, enabling efficient access to enantioenriched α-oxygenated ketones. This method exhibits a broad substrate scope, good functional group tolerance, high chemoselectivity, and excellent enantioselectivity.
Abstract
Light-driven decarboxylative cross-coupling has emerged as a pivotal platform for constructing C(sp3)–C(sp2) bonds in organic synthesis and medicinal chemistry. However, using two structurally dissimilar carboxylic acids as a feedstock to form chiral α-oxygenated ketones remains a considerable challenge due to side reactions such as decarboxylative reduction and homocoupling. Herein, we report for the first time a photoredox/nickel dual-catalyzed enantioselective decarboxylative acylation of α-hydroxy acid derivatives and aliphatic carboxylic acids, enabling efficient access to enantioenriched α-oxygenated ketones. This method exhibits a broad substrate scope, good functional group tolerance, high chemoselectivity, and excellent enantioselectivity (up to 99% e.e.). The advantage of this reaction is that it eliminates the need for metal reductants and the use of precious metal photocatalysts and utilizes renewable feedstocks. The use of a coiled-tube continuous-flow photoreactor can shorten the illumination time by half and obtain results comparable to those of a batch reaction. Furthermore, preliminary mechanistic experiments support a pathway in which photocatalytic decarboxylation generates α-oxy alkyl radical species, and the Ni(I)–alkyl intermediate activates the in situ–formed mixed anhydride followed by reductive elimination to give the product in enantiomerically pure form.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
| Filename | Description |
|---|---|
| anie202506424-sup-0001-SuppMat.pdf19.7 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1R. Uchida, K. Shiomi, J. Inokoshi, R. Masuma, T. Kawakubo, H. Tanaka, Y. Iwai, S. Omura, J. Antibiot. 1996, 49, 932–934.
- 2T. Tanaka, M. Kawase, S. Tani, Bioorg. Med. Chem. 2004, 12, 501–505.
- 3M. B. Andrus, E. J. Hicken, J. C. Stephens, D. K. Bedke, J. Org. Chem. 2006, 71, 8651–8654.
- 4D. A. Higgins, M. E. Pomianek, C. M. Kraml, R. K. Taylor, M. F. Semmelhack, B. L. Bassler, Nature 2007, 450, 883–886.
- 5P. Hoyos, J.-V. Sinisterra, F. Molinari, A. R. Alcántara, P. Domínguez De María, Acc. Chem. Res. 2010, 43, 288–299.
- 6W. Maneerat, W. Phakhodee, T. Ritthiwigrom, S. Cheenpracha, S. Deachathai, S. Laphookhieo, Phytochem. Lett. 2013, 6, 18–20.
- 7L. K. Jennings, L. P. Robertson, K. E. Rudolph, A. L. Munn, A. R. Carroll, J. Nat. Prod. 2019, 82, 2620–2626.
- 8S. Ramaswamy, A. C. Oehlschlager, Tetrahedron 1991, 47, 1145–1156.
- 9T. Ooi, D. Uraguchi, J. Morikawa, K. Maruoka, Org. Lett. 2000, 2, 2015–2017.
- 10H. Kajiro, S. Mitamura, A. Mori, T. Hiyama, Synlett 1998, 1998, 51–52.
10.1055/s-1998-3126 Google Scholar
- 11C. Palomo, M. Oiarbide, J. M. García, Chem. Soc. Rev. 2012, 41, 4150.
- 12F. A. Davis, B.-C. Chen, Chem. Rev. 1992, 92, 919–934.
- 13O. Onomura, H. Arimoto, Y. Matsumura, Y. Demizu, Tetrahedron Lett. 2007, 48, 8668–8672.
- 14P. Muthupandi, S. K. Alamsettia, G. Sekar, Chem. Commun. 2009, 3288–3290.
- 15R. S. Menon, A. T. Biju, V. Nair Beilstein, J. Org. Chem. 2016, 12, 444–461.
- 16Bugaut, X.; Glorius, F., Chem. Soc. Rev. 2012, 41, 3511–3522.
- 17D. Enders, U. Kallfass, Angew. Chem. Int. Ed. 2002, 41, 1743–1745.
10.1002/1521-3773(20020517)41:10<1743::AID-ANIE1743>3.0.CO;2-Q CAS PubMed Web of Science® Google Scholar
- 18D. Sharmaa, R. Chatterjeea, V. Dhayalan, R. Dandel, Synthesis 2022, 54, 4129–4166.
- 19L.-W. Xu, Y. Gao, J.-J. Yin, L. Lia, C.-G. Xia, Tetrahedron Lett. 2005, 46, 5317–5320.
- 20E. G. Delany, S. Connon, J. Org. Biomol. Chem. 2021, 19, 248–258.
- 21T. Soeta, S. Mizuno, Y. Hatanaka, Y. Ukaji, Tetrahedron 2017, 73, 3430–3437.
- 22S. M. Langdon, M. M. D. Wilde, K. Thai, M. Gravel, J. Am. Chem. Soc. 2014, 136, 7539–7542.
- 23K. E. Poremba, S. E. Dibrell, S. E. Reisman, ACS Catal. 2020, 10, 8237–8246.
- 24L.-M. Chen, S. E. Reisman, Acc. Chem. Res. 2024, 57, 751–762.
- 25E. L. Lucas, E. R. Jarvo, Nat. Rev. Chem. 2017, 1, 0065.
- 26J. L. Hofstra, A. H. Cherney, C. M. Ordner, S. E. Reisman, J. Am. Chem. Soc. 2018, 140, 139–142.
- 27A. H. Cherney, N. T. Kadunce, S. E. Reisman, J. Am. Chem. Soc. 2013, 135, 7442–7445.
- 28B.-B. Wu, J. Xu, K.-J. Bian, Q. Gao, X.-S. Wang, J. Am. Chem. Soc. 2022, 144, 6543–6550.
- 29J. Wu, H. Wu, X. Liu, Y. Zhang, G. Huang, C. Zhang, Org. Lett. 2022, 24, 4322–4327.
- 30D. Lin, Y. Chen, Z. Dong, P. Pei, H. Ji, L. Tai, L.-A. Chen, CCS Chem. 2023, 5, 1386–1397.
- 31H. Ji, D. Lin, L. Tai, X. Li, Y. Shi, Q. Han, L.-A. Chen, J. Am. Chem. Soc. 2022, 144, 23019–23029.
- 32Gao, Y.; Baran, P. S., Angew. Chem. Int. Ed. 2023, 62, e20231520.
- 33J. Yin, C. K. Maguire, N. Yasuda, A. P. J. Brunskill, A. Klapars, Org. Process Res. Dev. 2017, 21, 94–97.
- 34S. Ni, N. M. Padial, C. Kingston, J. C. Vantourout, D. C. Schmitt, J. T. Edwards, M. M. Kruszyk, R. R. Merchant, P. K. Mykhailiuk, B. B. Sanchez, S. Yang, M. A. Perry, G. M. Gallego, J. J. Mousseau, M. R. Collins, R. J. Cherney, P. S. Lebed, J. S. Chen, T. Qin, P. S. Baran, J. Am. Chem. Soc. 2019, 141, 6726–6739.
- 35Q. Lin, T. Diao, J. Am. Chem. Soc. 2019, 141, 17937–17948.
- 36H.-H. Zhang, H. Chen, C. Zhu, S. Yu, Sci. China Chem. 2020, 63, 637–647.
- 37W. Xu, T. XU, Acc. Chem. Res. 2024, 57, 1997–2011.
- 38Z. Li, C. Li, Y. Ding, H. Huo, Coord. Chem. Rev. 2022, 460, 214479.
- 39Y. Zhang, Z. Zhang, S. Zhu, L. Chu, Chin. J. Org. Chem. 2023, 43, 1023.
- 40P. Zhou, S. Lu, X. Wu, W. Zhong, T. XU, Org. Lett. 2023, 25, 2344–2348.
- 41Z. Zuo, H. Cong, W. Li, J. Choi, G. C. Fu, D. W. C. MacMillan, J. Am. Chem. Soc. 2016, 138, 1832–1835.
- 42C. Pezzetta, D. Bonifazi, R. W. M. Davidson, Org. Lett. 2019, 21, 8957–8961.
- 43Z. Li, L. Huan, J. Li, X. Shu, D. Zhong, W. Zhang, H. Huo, Angew. Chem. Int. Ed. 2023, 62, e202305889.
- 44J. Amani, E. Sodagar, G. A. Molander, Org. Lett. 2016, 18, 732–735.
- 45C. Li, J. Cheng, X. Wan, J. Li, W. Zu, Y. Xu, Y. Huang, H. Huo, J. Am. Chem. Soc. 2024, 146, 19909–19918.
- 46 Commercial availability of different chemicals is as follows: R–COOH (1687363), R–Br (212,517), R–CHO (117890), and R–COCl 1419. These data were acquired from the eMolecules database (https://www.emolecules.com/).
- 47M. S. Holm, S. Saravanamurugan, E. Taarning, Science 2010, 328, 602–605.
- 48S. Song, J. Qu, P. Han, M. J. Hülsey, G. Zhang, Y. Wang, S. Wang, D. Chen, J. Lu, N. Yan, Nat. Commun. 2020, 11, 4899.
- 49C.-Q. Deng, J. Deng, Green Chem. 2025, 27, 275–292.
- 50G. L. Brett, Q. He, C. Hammond, P. J. Miedziak, N. Dimitratos, M. Sankar, A. A. Herzing, M. Conte, J. A. Lopez-Sanchez, C. J. Kiely, D. W. Knight, S. H. Taylor, G. J. Hutchings, Angew. Chem. Int. Ed. 2011, 50, 10136–10139.
- 51C.-Q. Deng, J. Deng, Y. Fu, Green Chem. 2022, 24, 8477–8483.
- 52B. T. Boyle, N. W. Dow, C. B. Kelly, M. C. Bryan, D. W. C. MacMillan, Nature 2024, 631, 789–795.
- 53B. Zhang, J. He, Y. Gao, L. Levy, M. S. Oderinde, M. D. Palkowitz, T. G. M. Dhar, M. D. Mandler, M. R. Collins, D. C. Schmitt, P. N. Bolduc, T. Chen, S. Clementson, N. N. Petersen, G. Laudadio, C. Bi, Y. Kawamata, P. S. Baran, Nature 2023, 623, 745–751.
- 54A. Whyte, T. P. Yoon, Angew. Chem. Int. Ed. 2022, 61, e202213739.
- 55Y. Wei, J. Lam, T. Diao, Chem. Sci. 2021, 12, 11414–11419.
- 56X. Shu, L. Huan, Q. Huang, H. Huo, J. Am. Chem. Soc. 2020, 142, 19058–19064.
- 57L. Huan, X. Shu, W. Zu, D. Zhong, H. Huo, Nat. Commun. 2021, 12, 3536.
- 58X. Shu, D. Zhong, Q. Huang, L. Huan, H. Huo, Nat. Commun. 2023, 14, 125.
- 59L. Falivene, Z. Cao, A. Petta, L. Serra, A. Poater, R. Oliva, V. Scarano, L. Cavallo, Nat. Chem. 2019, 11, 872–879.
- 60M. A. Cismesia, T. P. Yoon, Chem. Sci. 2015, 6, 5426–5434.
- 61J. B. Diccianni, T. N. Diao, Trends Chem 2019, 1, 830–844.
- 62M. R. Aronoff, N. A. Bourjaily, K. A. Miller, Tetrahedron Lett. 2010, 51, 6375–6377.