Mechanosensitive Conjugated Oligoelectrolytes for Visualizing Temporal Changes in Live Cells
Dr. Ji-Yu Zhu
Department of Chemistry, National University of Singapore, Singapore, 117543 Singapore
Institute for Digital Molecular Analytics and Science, Nanyang Technological University, Singapore, 636921 Singapore
Search for more papers by this authorDr. Samuel J. W. Chan
Department of Chemistry, National University of Singapore, Singapore, 117543 Singapore
Institute for Functional Intelligent Materials, National University of Singapore, Singapore, 117544 Singapore
Search for more papers by this authorHongyue Cui
Mechanobiology Institute, National University of Singapore, Singapore, 117411 Singapore
Search for more papers by this authorDr. Alexander A. Mikhalovsky
Department of Chemistry and Biochemistry, Center for Polymers and Organic Solids, University of California Santa Barbara, Santa Barbara, California, 93106 USA
Search for more papers by this authorDr. Fernando L. Garcia
Institute for Functional Intelligent Materials, National University of Singapore, Singapore, 117544 Singapore
Search for more papers by this authorBrandon Yeow Wee Goh
Mechanobiology Institute, National University of Singapore, Singapore, 117411 Singapore
Search for more papers by this authorDr. Wilson Wee Mia Soh
Department of Chemistry, National University of Singapore, Singapore, 117543 Singapore
Search for more papers by this authorDr. Alex S. Moreland
Department of Chemistry and Biochemistry, Center for Polymers and Organic Solids, University of California Santa Barbara, Santa Barbara, California, 93106 USA
Search for more papers by this authorDr. Jakkarin Limwongyut
Department of Chemistry, National University of Singapore, Singapore, 117543 Singapore
Search for more papers by this authorDr. Sukanya Shyamasundar
Department of Anatomy, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, 117594 Singapore
Search for more papers by this authorDr. Ya Jun Wu
Department of Anatomy, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, 117594 Singapore
Search for more papers by this authorProf. Fengyi Liang
Department of Anatomy, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, 117594 Singapore
Search for more papers by this authorProf. Rong Li
Mechanobiology Institute, National University of Singapore, Singapore, 117411 Singapore
Search for more papers by this authorCorresponding Author
Prof. Guillermo C. Bazan
Department of Chemistry, National University of Singapore, Singapore, 117543 Singapore
Institute for Functional Intelligent Materials, National University of Singapore, Singapore, 117544 Singapore
Institute for Digital Molecular Analytics and Science, Nanyang Technological University, Singapore, 636921 Singapore
Department of Chemical and Biomolecular Engineering, National University of Singapore, Singapore, 117585 Singapore
E-mail: [email protected]
Search for more papers by this authorDr. Ji-Yu Zhu
Department of Chemistry, National University of Singapore, Singapore, 117543 Singapore
Institute for Digital Molecular Analytics and Science, Nanyang Technological University, Singapore, 636921 Singapore
Search for more papers by this authorDr. Samuel J. W. Chan
Department of Chemistry, National University of Singapore, Singapore, 117543 Singapore
Institute for Functional Intelligent Materials, National University of Singapore, Singapore, 117544 Singapore
Search for more papers by this authorHongyue Cui
Mechanobiology Institute, National University of Singapore, Singapore, 117411 Singapore
Search for more papers by this authorDr. Alexander A. Mikhalovsky
Department of Chemistry and Biochemistry, Center for Polymers and Organic Solids, University of California Santa Barbara, Santa Barbara, California, 93106 USA
Search for more papers by this authorDr. Fernando L. Garcia
Institute for Functional Intelligent Materials, National University of Singapore, Singapore, 117544 Singapore
Search for more papers by this authorBrandon Yeow Wee Goh
Mechanobiology Institute, National University of Singapore, Singapore, 117411 Singapore
Search for more papers by this authorDr. Wilson Wee Mia Soh
Department of Chemistry, National University of Singapore, Singapore, 117543 Singapore
Search for more papers by this authorDr. Alex S. Moreland
Department of Chemistry and Biochemistry, Center for Polymers and Organic Solids, University of California Santa Barbara, Santa Barbara, California, 93106 USA
Search for more papers by this authorDr. Jakkarin Limwongyut
Department of Chemistry, National University of Singapore, Singapore, 117543 Singapore
Search for more papers by this authorDr. Sukanya Shyamasundar
Department of Anatomy, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, 117594 Singapore
Search for more papers by this authorDr. Ya Jun Wu
Department of Anatomy, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, 117594 Singapore
Search for more papers by this authorProf. Fengyi Liang
Department of Anatomy, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, 117594 Singapore
Search for more papers by this authorProf. Rong Li
Mechanobiology Institute, National University of Singapore, Singapore, 117411 Singapore
Search for more papers by this authorCorresponding Author
Prof. Guillermo C. Bazan
Department of Chemistry, National University of Singapore, Singapore, 117543 Singapore
Institute for Functional Intelligent Materials, National University of Singapore, Singapore, 117544 Singapore
Institute for Digital Molecular Analytics and Science, Nanyang Technological University, Singapore, 636921 Singapore
Department of Chemical and Biomolecular Engineering, National University of Singapore, Singapore, 117585 Singapore
E-mail: [email protected]
Search for more papers by this authorGraphical Abstract
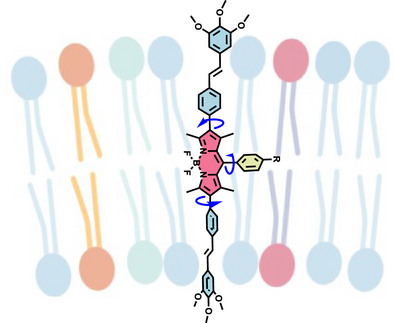
Mechanosensitive conjugated oligoelectrolytes (COEs) with integrated twistable bonds enable real-time imaging of membrane tension in lipid bilayers. Using fluorescence lifetime analysis, COE-BY probes reveal vesicle rigidity changes during endocytosis, providing insights into membrane mechanics at the subcellular level.
Abstract
Membrane-intercalating conjugated oligoelectrolytes (COEs) are lipid-bilayer-spanning molecules that serve as fluorescent dyes for bioimaging. However, COE emission has thus far only been capable of visualizing dye location and their preferential accumulation in different membrane-bound intracellular compartments. Herein, we report the first example of environmentally sensitive COEs for visualizing temporal changes in live cells, providing information on the physical properties of intracellular lipid bilayer membranes. The new COE-BY series is designed around a BODIPY central unit with a membrane-spanning topology and six cationic pendant groups ensuring solubility in aqueous media. These reporters feature high two-photon absorption cross section, NIR-II excitation capabilities under multiphoton excitation, and high dye brightness; all highly desirable photophysical features for bioimaging. The emission lifetime of the probes was sensitive to changes to both the lipid composition of model vesicle systems and membrane tension within cells, induced by either mechanical or osmotic stress. Using two-photon fluorescence lifetime imaging microscopy, it is possible to use the most efficient emitter, namely, COE-BYPhOC4, to image changes in the mechanical properties of intracellular membranes. We show that these COEs remain stably vesicle-bound within the endolysosomal pathway over extended periods, allowing for long-term monitoring of the associated biophysical changes of these vesicles over time.
Conflict of Interests
G.C.B. and J.-Y.Z. have filed for a provisional patent as employees of the National University of Singapore. The other authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in Supporting Information of this article.
Supporting Information
| Filename | Description |
|---|---|
| anie202506396-sup-0001-SuppMat.pdf4.1 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1M. S. Bretscher, Science 1973, 181, 622–629.
- 2D. Lingwood, K. Simons, Science 2010, 327, 46–50.
- 3P. Sharma, R. Varma, R. C. Sarasij, K. G. Ira, G. Krishnamoorthy, M. Rao, S. Mayor, Cell 2004, 116, 577–589.
- 4G. van Meer, D. R. Voelker, G. W. Feigenson, Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124.
- 5M. Schliwa, G. Woehlke, Nature 2003, 422, 759–765.
- 6D. J. Stephens, R. Pepperkok, Proc. Natl. Acad. Sci. 2001, 98, 4295–4298.
- 7S. Ray, A. Kassan, A. R. Busija, P. Rangamani, H. H. Patel, Am. J. Physiol. Cell Physiol. 2016, 310, C181–C192.
- 8H. Takakura, Y. Zhang, R. S. Erdmann, A. D. Thompson, Y. Lin, B. McNellis, F. Rivera-Molina, S.-n. Uno, M. Kamiya, Y. Urano, J. E. Rothman, J. Bewersdorf, A. Schepartz, D. Toomre, Nat. Biotechnol. 2017, 35, 773–780.
- 9C. Eggeling, C. Ringemann, R. Medda, G. Schwarzmann, K. Sandhoff, S. Polyakova, V. N. Belov, B. Hein, C. von Middendorff, A. Schönle, S. W. Hell, Nature 2009, 457, 1159–1162.
- 10C. Liu, X. Gao, J. Yuan, R. Zhang, TrAC Trends Anal. Chem. 2020, 133, 116092.
- 11M. Collot, P. Ashokkumar, H. Anton, E. Boutant, O. Faklaris, T. Galli, Y. Mély, L. Danglot, A. S. Klymchenko, Cell Chem. Biol. 2019, 26, 600–614.e7.
- 12M. Páez-Pérez, I. López-Duarte, A. Vyšniauskas, N. J. Brooks, M. K. Kuimova, Chem. Sci. 2021, 12, 2604–2613.
- 13C. Bernard, A. R. Carotenuto, N. M. Pugno, L. Deseri, M. Fraldi, Meccanica 2024, 59, 1231–1253.
- 14J. G. Carlton, H. Jones, U. S. Eggert, Nat. Rev. Mol. Cell Biol. 2020, 21, 151–166.
- 15N. C. Gauthier, M. A. Fardin, P. Roca-Cusachs, M. P. Sheetz, Proc. Natl. Acad. Sci. 2011, 108, 14467–14472.
- 16Y. Marcy, J. Prost, M.-F. Carlier, C. Sykes, Proc. Natl. Acad. Sci 2004, 101, 5992–5997.
- 17S. Sen, S. Subramanian, D. E. Discher, Biophys. J. 2005, 89, 3203–3213.
- 18R. M. Hochmuth, J. Biomech. 2000, 33, 15–22.
- 19J. Dai, M. P. Sheetz, Biophys. J. 1999, 77, 3363–3370.
- 20D. R. Stabley, C. Jurchenko, S. S. Marshall, K. S. Salaita, Nat. Methods 2012, 9, 64–67.
- 21C. Grashoff, B. D. Hoffman, M. D. Brenner, R. Zhou, M. Parsons, M. T. Yang, M. A. McLean, S. G. Sligar, C. S. Chen, T. Ha, M. A. Schwartz, Nature 2010, 466, 263–266.
- 22O. A. Kucherak, S. Oncul, Z. Darwich, D. A. Yushchenko, Y. Arntz, P. Didier, Y. Mély, A. S. Klymchenko, J. Am. Chem. Soc. 2010, 132, 4907–4916.
- 23R. Kreder, K. A. Pyrshev, Z. Darwich, O. A. Kucherak, Y. Mély, A. S. Klymchenko, ACS Chem. Biol. 2015, 10, 1435–1442.
- 24T. Baumgart, S. T. Hess, W. W. Webb, Nature 2003, 425, 821–824.
- 25A. Colom, E. Derivery, S. Soleimanpour, C. Tomba, M. D. Molin, N. Sakai, M. González-Gaitán, S. Matile, A. Roux, Nat. Chem. 2018, 10, 1118–1125.
- 26X. X. Chen, F. Bayard, N. Gonzalez-Sanchis, K. K. P. Pamungkas, N. Sakai, S. Matile, Angew. Chem. Int. Ed. 2023, 62, e202217868.
- 27M. Dal Molin, Q. Verolet, A. Colom, R. Letrun, E. Derivery, M. Gonzalez-Gaitan, E. Vauthey, A. Roux, N. Sakai, S. Matile, J. Am. Chem. Soc. 2015, 137, 568–571.
- 28A. Goujon, A. Colom, K. Strakova, V. Mercier, D. Mahecic, S. Manley, N. Sakai, A. Roux, S. Matile, J. Am. Chem. Soc. 2019, 141, 3380–3384.
- 29F. Piazzolla, V. Mercier, L. Assies, N. Sakai, A. Roux, S. Matile, Angew. Chem. Int. Ed. 2021, 133, 12366–12371.
10.1002/ange.202016105 Google Scholar
- 30C. Roffay, G. Molinard, K. Kim, M. Urbanska, V. Andrade, V. Barbarasa, P. Nowak, V. Mercier, J. García-Calvo, S. Matile, R. Loewith, A. Echard, J. Guck, M. Lenz, A. Roux, Proc. Natl. Acad. Sci. 2021, 118, e2103228118.
- 31D. Mahecic, L. Carlini, T. Kleele, A. Colom, A. Goujon, S. Matile, A. Roux, S. Manley, Cell Rep. 2021, 35, 108947.
- 32V. Mercier, J. Larios, G. Molinard, A. Goujon, S. Matile, J. Gruenberg, A. Roux, Nat. Cell Biol. 2020, 22, 947–959.
- 33K. Oglęcka, P. Rangamani, B. Liedberg, R. S. Kraut, A. N. Parikh, eLife 2014, 3, e03695.
- 34L. E. Garner, J. Park, S. M. Dyar, A. Chworos, J. J. Sumner, G. C. Bazan, J. Am. Chem. Soc. 2010, 132, 10042–10052.
- 35D. Ding, K.-Y. Pu, K. Li, B. Liu, Chem. Commun. 2011, 47, 9837.
- 36H. Hou, X. Chen, A. W. Thomas, C. Catania, N. D. Kirchhofer, L. E. Garner, A. Han, G. C. Bazan, Adv. Mater. 2013, 25, 1593–1597.
- 37L. Zhou, F. Lv, L. Liu, S. Wang, Acc. Chem. Res. 2019, 52, 3211–3222.
- 38L. Cai, R. Zhan, K.-Y. Pu, X. Qi, H. Zhang, W. Huang, B. Liu, Anal. Chem. 2011, 83, 7849–7855.
- 39Q. Feng, Z. Zhang, Q. Yuan, M. Yang, C. Zhang, Y. Tang, Sens. Actuators B Chem 2020, 312, 127981.
- 40S. J. W. Chan, J.-Y. Zhu, W. W. Mia Soh, G. C. Bazan, J. Am. Chem. Soc. 2024, 146, 660–667.
- 41Y. Wang, E. Y. Chi, D. O. Natvig, K. S. Schanze, D. G. Whitten, ACS Appl. Mater. Inter. 2013, 5, 4555–4561.
- 42S. J. W. Chan, K. Zhang, J.-Y. Zhu, G. C. Bazan, Chem. Eur. J. 2023, 29, e202203803.
- 43P. Gwozdzinska, R. Pawlowska, J. Milczarek, L. E. Garner, A. W. Thomas, G. C. Bazan, A. Chworos, Chem. Commun. 2014, 50, 14859–14861.
- 44C. Zhou, S. J. Cox-Vázquez, G. W. N. Chia, R. J. Vázquez, H. Y. Lai, S. J. W. Chan, J. Limwongyut, G. C. Bazan, Sci. Adv. 2023, 9, eade2996.
- 45J.-Y. Zhu, G. C. Bazan, Cell Rep. Phys. Sci. 2023, 4, 101429.
- 46J.-Y. Zhu, A. Mikhailovsky, S. J. W. Chan, A. Moreland, J. Limwongyut, N. Guarrotxena, G. C. Bazan, Adv. Funct. Mater. 2023, 33, 2305962.
- 47C. Zhou, Z. Li, Z. Zhu, G. W. N. Chia, A. Mikhailovsky, R. J. Vázquez, S. J. W. Chan, K. Li, B. Liu, G. C. Bazan, Adv. Mater. 2022, 34, 2201989.
- 48L. Wang, Y. Xiao, W. Tian, L. Deng, J. Am. Chem. Soc. 2013, 135, 2903–2906.
- 49I. López-Duarte, T. T. Vu, M. A. Izquierdo, J. A. Bull, M. K. Kuimova, Chem. Commun. 2014, 50, 5282–5284.
- 50P. Ashokkumar, A. H. Ashoka, M. Collot, A. Das, A. S. Klymchenko, Chem. Commun. 2019, 55, 6902–6905.
- 51M. K. Kuimova, G. Yahioglu, J. A. Levitt, K. Suhling, J. Am. Chem. Soc. 2008, 130, 6672–6673.
- 52J. T. Mika, A. J. Thompson, M. R. Dent, N. J. Brooks, J. Michiels, J. Hofkens, M. K. Kuimova, Biophys. J. 2016, 111, 1528–1540.
- 53X. Liu, W. Chi, Q. Qiao, S. V. Kokate, E. P. Cabrera, Z. Xu, X. Liu, Y.-T. Chang, ACS Sens. 2020, 5, 731–739.
- 54T. Kowada, H. Maeda, K. Kikuchi, Chem. Soc. Rev. 2015, 44, 4953–4972.
- 55J. Steinkühler, E. Sezgin, I. Urbančič, C. Eggeling, R. Dimova, Commun. Biol. 2019, 2, 337.
- 56C. Bissig, J. Gruenberg, Cold Spring Harb. Perspect. Biol. 2013, 5, a016816.
- 57K. Sobo, J. Chevallier, R. G. Parton, J. Gruenberg, F. G. van der Goot, PLoS One 2007, 2, e391.
- 58B. Diaz-Rohrer, I. Castello-Serrano, S. H. Chan, H.-Y. Wang, C. R. Shurer, K. R. Levental, I. Levental, Proc. Natl. Acad. Sci. 2023, 120, e2207461120.
- 59H. S. Jung, J. Han, H. Shi, S. Koo, H. Singh, H.-J. Kim, J. L. Sessler, J. Y. Lee, J.-H. Kim, J. S. Kim, J. Am. Chem. Soc. 2017, 139, 7595–7602.
- 60Z. Zhang, D. Yomo, C. Gradinaru, Biochim. Biophys. Acta Biomembr. 2017, 1859, 1242–1253.
- 61W. Becker, Advanced time-correlated single photon counting techniques,Springer Series in Chemical Physics, Vol. 81, Springer, Berlin, Heidelberg 2005.
10.1007/3-540-28882-1 Google Scholar
- 62H. C. Gerritsen, M. A. H. Asselbergs, A. V. Agronskaia, W. G. J. H. M. Van Sark, J. Microsc. 2002, 206, 218–224.
- 63R. M. Williams, W. R. Zipfel, W. W. Webb, Curr. Opin. Chem. Biol. 2001, 5, 603–608.
- 64D. A. Oulianov, I. V. Tomov, A. S. Dvornikov, P. M. Rentzepis, Opt. Commun. 2001, 191, 235–243.
- 65C. Xu, W. W. Webb, J. Opt. Soc. Am. B 1996, 13, 481.
- 66L. V. Albrecht, N. Tejeda-Muñoz, E. M. De Robertis, STAR Protoc 2020, 1, 100132.
- 67B. Zhitomirsky, H. Farber, Y. G. Assaraf, J. Cell. Mol. Med. 2018, 22, 2131–2141.
- 68M. Nagano, J. Y. Toshima, D. E. Siekhaus, J. Toshima, Commun. Biol. 2019, 2, 419.
- 69J. M. Hyttinen, M. Niittykoski, A. Salminen, K. Kaarniranta, Biochim. Biophys. Acta Mol. Cell Res. 2013, 1833, 503–510.
- 70P. Saftig, J. Klumperman, Nat. Rev. Mol. Cell Biol. 2009, 10, 623–635.
- 71C. C. Yap, L. Digilio, L. P. McMahon, T. Wang, B. Winckler, J. Neurosci. Res. 2022, 42, 4415–4434.
- 72S. J. W. Chan, J.-Y. Zhu, B. R. P. Yip, S. Shyamasundar, G. C. Bazan, Adv. Mater. 2025, 37, 2411329.
- 73A. S. Klymchenko, Acc. Chem. Res. 2023, 56, 1–12.
- 74K. Suhling, L. M. Hirvonen, J. A. Levitt, P.-H. Chung, C. Tregidgo, A. Le Marois, D. A. Rusakov, K. Zheng, S. Ameer-Beg, S. Poland, S. Coelho, R. Henderson, N. Krstajic, Medical Photonics 2015, 27, 3–40.
- 75A. Magni, G. Bondelli, G. M. Paternò, S. Sardar, V. Sesti, C. D'Andrea, C. Bertarelli, G. Lanzani, Phys. Chem. Chem. Phys. 2022, 24, 8716–8723.
- 76L. Malacrida, S. Ranjit, D. M. Jameson, E. Gratton, Annu. Rev. Biophys. 2021, 50, 575–593.
- 77S. Ranjit, L. Malacrida, D. M. Jameson, E. Gratton, Nat. Protoc. 2018, 13, 1979–2004.
- 78H. Jiang, S. X. Sun, Biophys. J. 2013, 105, 609–619.
- 79A. J. Lomakin, C. J. Cattin, D. Cuvelier, Z. Alraies, M. Molina, G. P. F. Nader, N. Srivastava, P. J. Sáez, J. M. Garcia-Arcos, I. Y. Zhitnyak, A. Bhargava, M. K. Driscoll, E. S. Welf, R. Fiolka, R. J. Petrie, N. S. De Silva, J. M. González-Granado, N. Manel, A. M. Lennon-Duménil, D. J. Müller, M. Piel, Science 2020, 370, eaba2894.
- 80M. Le Berre, E. Zlotek-Zlotkiewicz, D. Bonazzi, F. Lautenschlaeger, M. Piel, in Methods in Cell Biology, Vol. 121, (Eds.: M. Piel, M. Théry), Academic Press, Cambridge, Massachusetts 2014, pp. 213–229.
- 81Y.-J. Liu, M. Le Berre, F. Lautenschlaeger, P. Maiuri, A. Callan-Jones, M. Heuzé, T. Takaki, R. Voituriez, M. Piel, Cell 2015, 160, 659–672.
- 82L. Venkova, A. S. Vishen, S. Lembo, N. Srivastava, B. Duchamp, A. Ruppel, A. Williart, S. Vassilopoulos, A. Deslys, J. M. Garcia Arcos, A. Diz-Muñoz, M. Balland, J.-F. Joanny, D. Cuvelier, P. Sens, M. Piel, eLife 2022, 11, e72381.
- 83G. T. Charras, M. Coughlin, T. J. Mitchison, L. Mahadevan, Biophys. J. 2008, 94, 1836–1853.
- 84R. Gagescu, N. Demaurex, R. G. Parton, W. Hunziker, L. A. Huber, J. Gruenberg, Mol. Biol. Cell 2000, 11, 2775–2791.
- 85S. Mukherjee, T. T. Soe, F. R. Maxfield, J. Cell Biol. 1999, 144, 1271–1284.
- 86N. A. Bright, L. J. Davis, J. P. Luzio, Curr. Biol. 2016, 26, 2233–2245.