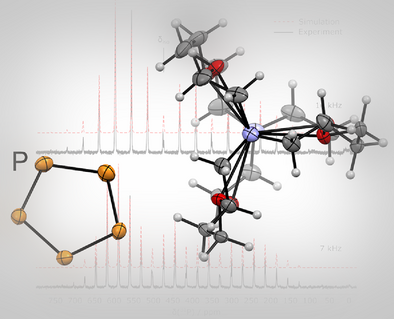
Cyclo-P5− Revisited: The Surprisingly Stable Uncoordinated Pentaphospholide Anion
In memoriam Prof. Dr. Peter Jutzi
Graphical Abstract
The intriguing cyclo-P5− anion, which is isolobal to the well-known cyclopentadienide anion, was characterized for the first time crystallographically in the salts [M([2.2.2]cryptand)][cyclo-P5] (M = Na, K). The compounds were further characterized by UV/Vis spectroscopy in solution and in the solid state by Raman and 31P MAS NMR spectroscopy.
Abstract
The addition of [2.2.2]cryptand to alkali metal heptaphosphides M3P7 (M = Na, K) leads to the formation of [M([2.2.2]cryptand)][cyclo-P5] salts. Although the cyclo-P5− anion is spectroscopically known since the 1980s, it was so far prepared and handled only in solution. By complexing the alkali metal cation with [2.2.2]cryptand, the product was found to be surprisingly stable in the solid state. Cyclo-P5−, which is isolobal to the well-known cyclopentadienide anion, was now characterized crystallographically for the first time in its uncoordinated form and in the presence of the weakly coordinating cations [M(2.2.2-cryptand)]+ (M = Na, K). The structural elucidation proves its planar D5 h symmetry, not only as ligand in different sandwich complexes but also in its uncoordinated form. Cyclo-P5− was further characterized by UV/Visis spectroscopy in solution and in the solid state by Raman and 31P MAS NMR spectroscopy. The reaction of [Na([2.2.2]cryptand)][cyclo-P5] with LiCp* and FeCl2 yields the ferrocene derivative [Cp*Fe(cyclo-P5)].
The electron-rich, aromatic cyclopentadienide anion C5H5− (A, Figure 1) is undoubtedly one of the most versatile ligands in organometallic and coordination chemistry and plays an important role in both fundamental research and practical applications. Taking the isolobal principle into account, the related monocyclic compounds cyclo-Pn5− (B–F, Figure 1) are known for all pnictogens (Pn). Interestingly, among the series B–F, only the cyclo-N5− (B) has so far been characterized crystallographically in its uncoordinated form.[1-3] In the first reports on the salt (N5)6(H3O)3(NH4)4Cl by Ming Lu and co-workers in 2017, hydrogen bonding between the ammonium and oxonium ions and the nitrogen atoms of the pentazolate anion was also observed.[1]
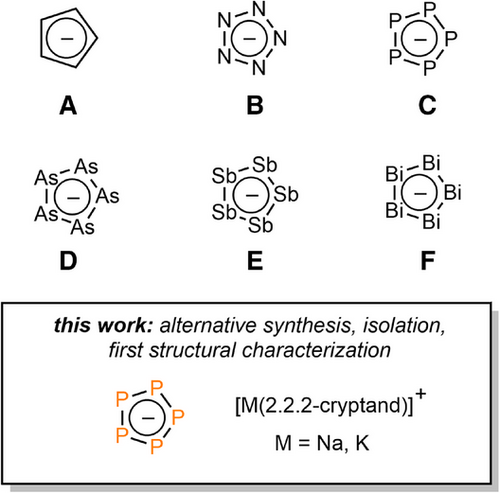
Since the discovery of cyclo-P5− by Baudler and co-worker, it was generally accepted that C exists only in solution, as it oligomerizes rapidly upon removing the solvent.[4-6] Nevertheless, the evidence of its aromatic and highly symmetric structure is evident from the corresponding 31P NMR spectrum, which shows only one significantly deshielded singlet resonance with a chemical shift at δ(ppm) = 470.0.[4] Ab initio calculations of the cyclo-P5− anion also confirmed the D5 h planar cyclic structure with a P─P bond length of around 2.093 Å.[7, 8] Several computational studies on the electronic structure and aromaticity of cyclo-P5−, especially in comparison with its isolobal analogue C5H5−, have been reported in the last decades.[9-14] Moreover, cyclo-P5− was investigated intensively as ligand in sandwich complexes.[15-20] The majority of such coordination compounds were synthesized by activation of white phosphorus with transition metal complexes to access, for example, the well-known ferrocene derivative [Cp*Fe(cyclo-P5)], the related Ru(II) complex [Cp*Ru(cyclo-P5)], or the carbon-free sandwich complex [Ti(cyclo-P5)2]2−.[16-19] [Cp*Fe(cyclo-P5)] was shown to act as cyclo-P5−-transfer agent yielding, for example, the corresponding osmium complex.[20] Moreover, the generation of cyclo-P5− in solution has opened up the possibility to transfer this anionic homocycle as ligand into coordination compounds.[21] The presence of energetically and sterically accessible lone pairs at each phosphorus atom further led to the synthesis of polynuclear and supramolecular coordination compounds,[22-24] while investigations on their reactivity towards nucleophiles and electrophiles were impressively demonstrated by Scheer, Roesky and co-workers.[25-27]
In contrast, much less is known about cyclo-As5− and cyclo-Sb5−. The cyclopentaarsenide anion D has been generated starting from As4 and was isolated as both triple-decker and sandwich-complexes, while the pentastibacyclopentadienide anion E is accessible from cyclo-tBu4Sb4.[28, 29] Again, E was so far only characterized crystallographically as a triple-decker Mo-sandwich complex.[29] Finally, Dehnen and co-workers recently reported on the successful generation of cyclo-Bi5− (F), the heaviest analogue of C5H5−, and its crystallographic characterization as the anionic Co-complex [IMes2Co2Bi5]− (IMes: bis(1,3-(2,4,6-trimethylphenyl))-imidazol-2-ylidene).[30] During our investigation on the reactivity of C towards C≡E (E = C, N, P) triple bonds,[31] we noticed that the chemistry of cyclo-P5− solutions must be revisited, as we could now isolate and fully characterize C as its [M(2.2.2-cryptand)]+ (M = Na, K) salts.
Several approaches to generate the cyclo-P5− ion in solution are presently known.[4, 5, 32, 33] Generally, cyclo-P5− as well as other polyphosphides (P73−, P162−) form upon activation of P4 or Pred with alkali metals or strong nucleophiles.[34] In the seminal report by Baudler and co-workers from 1987, cyclo-P5− was detected among several other products, which were formed when reacting P4 and Na in refluxing diglyme.[4] The same group later reported that 0.01 M stock-solutions of pure [Na(solvent)n][cyclo-P5] (yield based on P4 is around 12%) can be obtained upon activation of P4 with Na in THF.[5] Most importantly, the precipitation of other sodium polyphosphides is facilitated by adding 18-crown-6 and the solution of cyclo-P5− in diglyme can be used for follow-up chemistry. Two decades later, Miluykov and Hey-Hawkins demonstrated that P4 reacts in the presence of dibenzo-18-crown-6 with sodium in refluxing diglyme to give [Na(diglyme)n][cyclo-P5] in 25% spectroscopic yield, while the second main product of the reaction is Na3P7, which is insoluble and precipitates from the reaction mixture.[32] Nevertheless, all reports so far describe that cyclo-P5− is only stable in solution and decomposes upon cooling or when the solvent is removed under vacuum.
With the aim in mind to find a cyclo-P5− source, that is thermodynamically and kinetically stable, easily accessible and useable, we investigated a novel synthetic route towards this anionic, aromatic phosphorus cycle using [2.2.2]cryptand for the complexation of the alkali metal cation. In fact, Milyukov et al. suggested a pathway towards cyclo-P5−, in which higher polyphosphides, for instance Na3P7 or Na2P16, are involved. As mentioned above, such species can indeed be detected as byproducts during the synthesis of cyclo-P5−.[32, 33] Moreover, Goicoechea and co-worker reported on the preparation of [K([2.2.2]crypt)]2[Co(η5-P5){η2-P2H(mes)}]2.[34] This coordination compound is formed upon reaction of K3P7 with [Co(mes)2(PEt2Ph)2] (mes = 2,4,6-trimethylphenyl) and contains both a cyclo-P5− and a diphosphene ligand. The alkali metal heptaphosphides M3P7(dme)x (M = Na (1a), K(1b)) are independently accessible quantitatively from red (Pred) or white phosphorus (P4) and sodium in the presence of naphthalene.[35]
Motivated by these findings, stoichiometric amounts of 1a and 1b were first reacted with [2.2.2]cryptand under reflux in different ethers (THF, diglyme). Much to our surprise, the 31P NMR spectra of the yellow-orange suspensions only revealed a single resonance at δ = 468.0 ppm after heating, while the reaction time depends on the boiling point of the solvent. In THF, the highest yield was reached after 72 h, while for diglyme the reaction is already completed after 15 h. This resonance can be attributed to the formation of the cyclo-P5− anion as only soluble phosphorus species (Scheme 1a; for the insoluble residue see SI). Interestingly, we noticed that the same reactivity is observed when stoichiometric amounts of white phosphorus, potassium and [2.2.2]cryptand in THF are refluxed for 15 h (Scheme 1b).
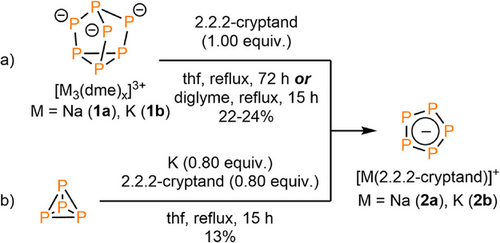
The filtered solutions were subsequently layered with pentane. After storing the samples for 3 days at T = –20°C in the freezer, [M([2.2.2]cryptand)][cyclo-P5] (M = Na (2a), K (2b)) crystallized as light-yellow crystalline blocks, suitable for single crystal X-ray diffraction. Compound 2a crystalized from the diglyme solution in the triclinic space group , while 2b crystalized in the monoclinic space group P21/n. The molecular structures of 2a and 2b in the crystals are depicted in Figures 2 and 3, along with selected bond lengths and angles (For 2a(thf): see Figure S2
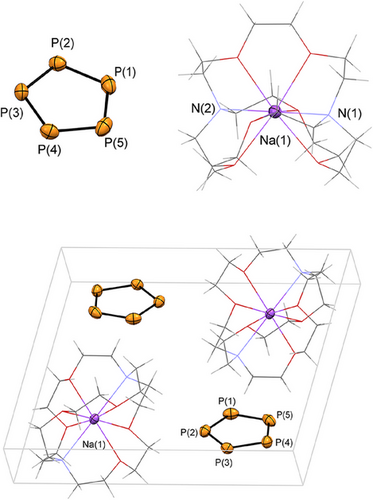

Much to our delight, the crystallographic characterization of the products confirmed the formation of 2a and 2b and, for the first time, the stability of the cyclo-P5− anion in the solid state. The packed unit cell shows neither significant anion-cation, nor anion-anion interactions but only the naked cyclo-P5− separated from the weakly coordinating cation. As anticipated spectroscopically and by means of computational chemistry, the isolated cyclo-P5− anion reveals a planar structure, which is in accordance with the solid-state structures of the isolobal cyclopentadiene anion and the valence isoelectronic cyclo-N5− anion. The average P─P bond length in the five-membered phosphorus cycle is 2.074 Å for 2a and 2.085 Å for 2b, which is between common P─P-single (2.21 Å) and P═P-double (2.02 Å) bonds.[36] The average P─P─P angles are 108.00°, as expected for a regular pentagon. Moreover, the P─P bond lengths are in the range of the experimentally determined P─P distances in reported iron and ruthenium mono-P5 complexes (2.07–2.12 Å).[16, 18] The P─P─P─P torsion angles are below 0.05° (2a), 1.22°(2a(thf)) and 0.26° (2b).
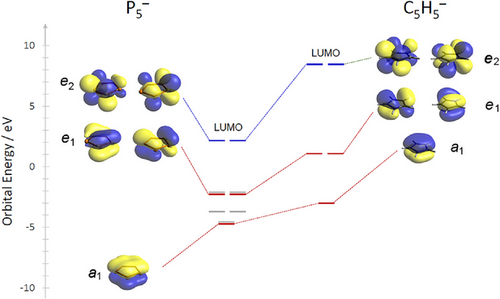
The geometry optimization of the cyclo-P5− anion performed at the PBE0-D3/def2-TZVP level indeed reveals a D5 h planar structure of the phosphorus cycle with P─P distances of 2.0899 Å and P─P─P angles of 108°, which are perfectly in line with the experimentally observed data. The calculated 31P NMR chemical shift of δ = 516 ppm for the cyclo-P5− anion is also close to the experimental value (see ESI). Figure 4 represents an overview of selected frontier orbitals of the pentaphospholide anion in comparison to the isolobal cyclopentadienide anion. As already reported in literature,[17] the cyclo-P5− anion acts in the corresponding transition metal π-complexes as a less good σ- and π-donor, but a much better δ-acceptor ligand than its Cp− analog (HOMO−1, e1: E = −2.387 eV; LUMO, e2: E = 2.310 eV).[37] Most strikingly, the crystallized salts 2a and 2b can be isolated by filtration, followed by vacuum drying. The resulting pale yellow-orange powders can be stored under argon at room temperature over several weeks.
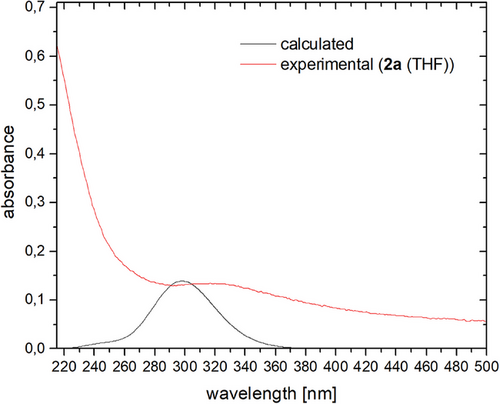
Moreover, the [M([2.2.2]cryptand)][cyclo-P5] salts can be re-dissolved in organic solvents, such as THF and CH2Cl2. While 2a/b are stable in THF for several weeks, oligomerization/decomposition can be observed after several hours in CH2Cl2. In this case, an insoluble orange residue is formed, which could not be identified by means of 31P NMR spectroscopy. This residue can, however, be filtered off, while the remaining solution contains only 2a/b. Supported by computational data, the isolation of the [M([2.2.2]cryptand)][cyclo-P5] salts allows for the first time additional characterizations of such compounds, both in solution and in the solid state. The UV/Vis spectrum of 2a in THF shows a broad band between λ = 280 nm and λ = 400 nm (2b: λmax = 270 nm), which can be attributed mainly to π→π* transitions in the cyclo-P5− anion (Figures 4 and 5 and SI).[38] Similar concentration-dependent spectra were observed by Baudler and co-worker for NaP5/[18]crown-6/THF solutions.[5]
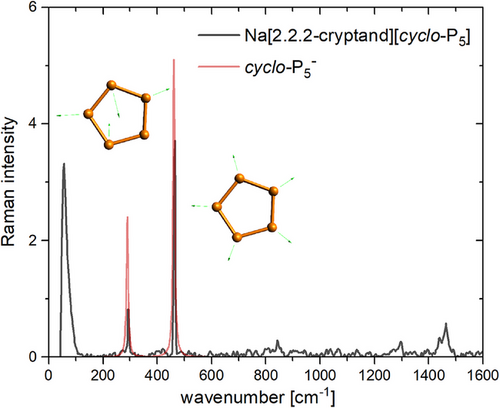
The powder Raman spectrum of 2a shows two characteristic bands for the cyclo-P5− anion at 464 cm−1 (Figure 6: ring breathing vibration, calculated value: 461 cm−1) and at 294 cm−1 (Figure 6; ring distortion vibration, calculated value: 289 cm−1).
Compound 2a was also characterized by means of solid-state 31P NMR spectroscopy under magic-angle spinning (MAS). The spectra, measured at different spin rates (7 and 10 kHz), are depicted in Figure 7.
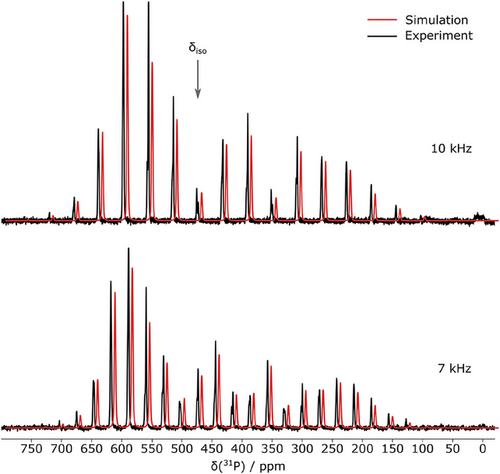
Both spectra show a mutual isotropic central band at δiso = 473.3 ppm with a number of rotational sidebands separated by the respective MAS frequency of 7 and 10 kHz, respectively. Spectral simulation revealed a chemical shift anisotropy (CSA) with a reduced anisotropy (δzz − δiso) of −320.0 ppm and no asymmetry (η = 0). The quality of the multi-frequency fit and the absence of any additional resonances is in line with the observation from crystallographic data that all 31P atoms in each cyclo-P5− ring as well as both rings in the unit cell are virtually identical, and further displays the high symmetry and purity of the compound. Each signal (i.e., central band and sidebands) features a splitting pattern of ∼2.4 ppm (570 Hz), which lies in the same order of magnitude as 31P-31P 1J-couplings reported in literature.[39] Note, that in the case of cyclo-P5−, the expected J-coupling pattern is non-trivial due to the near-isochronicity of the five 31P nuclei. Perfect isochronicity would render any J-coupling invisible; however, small inequivalences in each nucleus's proximity likely introduce chemical shift deviations of similar magnitude as the observed splitting. This could lead to the observed splitting in an “intermediate-coupling” situation.[40]
In view of the rather high stability of 2a/b both in the solid state and in solution, we examined the preparation of corresponding coordination compounds. For this purpose, we envisaged the synthesis of the known ferrocene derivative [Cp*Fe(cyclo-P5)] (3) that shows a chemical shift in the 31P{1H} NMR spectrum at δ = 151.5 ppm. Baudler and co-worker reported on its synthesis from Li(cyclo-P5), LiCp* and FeCl2.[5] Much to our delight, we found a similar reactivity of 2a/b compared to the Li(cyclo-P5), generated in situ (Scheme 2, and SI).
In conclusion, we have developed two new synthetic routes for alkali metal salts of the cyclo-pentaphospholide anion. By using [2.2.2]cryptand, it was possible to generate the cyclo-P5−-anion from the alkali metal heptaphosphides M3P7 (M = Na, K). A similar reactivity was observed when using stoichiometric amounts of white phosphorus, potassium and [2.2.2]cryptand in THF. The isolation as the salts [M(2.2.2-cryptand)][cyclo-P5] (M = Na, K) led for the first time to a crystallographic characterization of both compounds. The fully inorganic cyclopentadienide derivates were additionally investigated by means of theoretical and experimental methods, such as Raman-, IR-, UV/Vis-, as well as solid-state 31P MAS NMR spectroscopy. The stability of the salts [M([2.2.2]cryptand)][cyclo-P5] (M = Na, K) allows the handling of the cyclo-P5− anion in its uncoordinated form in organic solvents, such as THF and CH2Cl2. Moreover, the reaction of [Na([2.2.2]cryptand)][cyclo-P5] with LiCp* and FeCl2 yields the ferrocene derivative [Cp*Fe(cyclo-P5)].
Supporting Information
The authors have cited additional references within the Supporting Information.[41-65] The Supporting Information includes detailed syntheses for all the reported compounds, analytical and spectroscopic data and a summary of the computational techniques used and relevant results.
Acknowledgements
The authors gratefully acknowledge Freie Universität Berlin for financial support, G. Drendel and the NMR Core Facility team of the Freie Universität Berlin for their help in recording the 31P MAS NMR spectra, ITMZ at the University of Rostock for access to the Cluster Computer and especially M. Willert for technical support.
Open access funding enabled and organized by Projekt DEAL.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.