PCET Photodecarboxylation for SN2’-Type Alkylation of gem-Dichlorocyclobutenones
Graphical Abstract
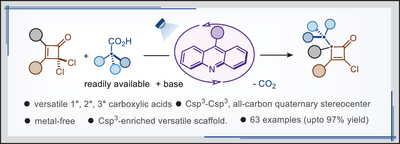
A versatile radical SN2’-type alkylation of gem-dichlorocyclobutenones is reported by photodecarboxylation of free carboxylic acids via proton-coupled electron transfer (PCET). This mild and scalable Csp3─Csp3 bond forming alkylation method enables efficient access to Csp3-enriched 4-alkylated α-chlorocyclobutenones with excellent functional group tolerance, employing carboxylic acid-based drugs, fatty acids, and steroids.
Abstract
Metal-catalyzed nucleophilic SN2’-type alkylation of gem-dichlorocyclobutenones with organometallic reagents remained unsuccessful. Herein, we report an acridine-catalyzed radical SN2’ alkylation of both β-alkyl and β-aryl substituted gem-dichlorocyclobutenones with free carboxylic acids. In this process, Csp3-enriched 4-alkylated 2-chlorocyclobutenones are obtained with commendable functionality tolerance. A PCET photodecarboxylation enables for the first time SN2’-type alkylation of gem-dichlorocyclobutenones with β-H-containing carboxylic acids, addressing several challenges. This mild and scalable Csp3─Csp3 bond-forging alkylation applies to the functionalization of natural product-derived gem-dichlorocyclobutenones with carboxylic acid drugs, fatty acids, and steroids. The α-chlorocyclobutenone product is further exploited in divergent organic synthesis via postsynthetic modification.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.