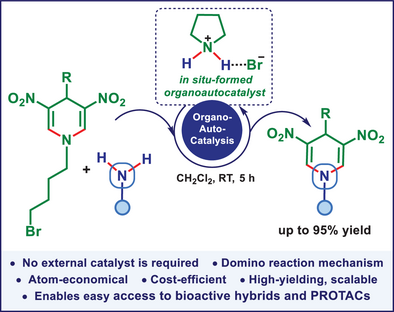
Development of an Organoautocatalyzed Double σ-Bond C(sp2)-N Transamination Metathesis Reaction
Dedicated to Professor Kensō Soai on the occasion of his 75th birthday
Graphical Abstract
Organoautocatalyzed transamination metathesis of cyclic tertiary amines is disclosed as a high-yielding, scalable reaction that proceeds under mild, catalyst-free conditions. It operates via a multi-step domino reaction mechanism, where an in situ-formed pyrrolidinium salt functions as a HBD organoautocatalyst. The reaction opens the door for the efficient synthesis of novel N-substituted DNDHPs of interest in both life and materials sciences.
Abstract
The transamination reaction, which involves the conversion of one amine to another, traditionally relies on biological enzyme catalysts. Although chemists have recently developed a few transition metal-catalyzed methods, mimicking these enzymes to interconvert amine groups in acyclic substrates via transamination metathesis of a single C(sp2)─N bond, transamination of cyclic tertiary amines has remained a challenge in synthetic chemistry. Here, we present the development of organoautocatalyzed transamination metathesis of two C(sp2)─N bonds in a cyclic substrate that allows for the challenging transformation to take place with up to 95% yield under exceptionally mild reaction conditions at room temperature without external catalysts and/or additives. The reaction mechanism has been studied in detail through time-resolved 1H-NMR, 2D NMR, and computational methods. Remarkably, in situ-formed pyrrolidinium salt acts as a hydrogen bond donor (HBD) organoautocatalyst in this multi-step domino process. The new organoautocatalyzed methodology gives environmentally friendly, atom-economical, straightforward, and rapid access to N-substituted 3,5-dinitro-1,4-dihydropyridines (DNDHPs), thus offering facile entry to privileged bioactive compounds.
Introduction
Over myriads of years of evolution, nature has developed intricate enzyme catalysts, accomplishing the transfer of amino-nitrogen from α-amino acids to α-keto-acids to produce their corresponding amino acids. This double displacement reaction is a transamination metathesis reaction, whereby two compound reactants AB and CD result in products of AC and BD.[1] The ever-increasing practical importance of carbon-heteroatom bond metathesis reactions is underlined by recent examples of transition metal-catalyzed C(sp2)─S and C(sp2)─P bond metathesis processes by Morandi and coworkers (Figure 1a).[2-5] In an attempt to follow nature's efficient examples, chemists have also developed a few biomimetic methods for transamination metathesis of C(sp2)─N bonds in the laboratory. Existing examples of single σ-bond C(sp2)─N metathesis include catalytic secondary amide transamidation reactions,[6, 7] transamination of substituted guanidines,[8] and of dimethylaminomethyleneoxindoles.[9] Although some rare advances have been made with regard to the transamination of N-substituted acyclic amines, which is emerging as a highly beneficial synthetic process, transamination of N-substituted cyclic amines has remained challenging. However, cyclic amines are among the most valuable classes of compounds in chemistry and are ubiquitous in bioactive compounds and pharmaceuticals. In particular, compounds comprising a 1,4-dihydropyridine (1,4-DHP) scaffold play a central role in the development of pharmaceuticals,[10-14] model compounds of redox coenzyme nicotinamide adenine dinucleotide (NADH), and as hydride sources in synthetic chemistry.[15, 16] Remarkably, 2,6-unsubstituted 1,4-DHPs exhibit strong blue fluorescence,[17, 18] although 2,6-dimethyl-1,4-DHPs are not fluorescent. These properties of 2,6-unsubstituted 1,4-DHP derivatives open new perspectives for their applications as photoelectronic functional materials.[19]
Furthermore, 4-arylated N-substituted 1,4-DHPs have drawn much recent attention in medicinal chemistry, finding applications as anti-asthmatic[20] or anti-cancer[21] agents and were also reported as potential remedies for overcoming multidrug resistance.[22] Undoubtedly, through variation of substituents at nitrogen in 1,4-DHPs, new compounds with strikingly different and unprecedented properties can be obtained. Due to this and constantly increasing applications, N-substituted 1,4-DHP has become a privileged scaffold for the synthetic methodology developed over the past few decades.[23-26] Many advances have been made toward N-substituted 1,4-DHPs synthesis via modified Hantzsch reactions.[27] However, existing methods require the synthesis of each individual N-substituted 1,4-DHP through Hantzsch-type reactions, to generate the compound libraries, essential for SARs (structure-activity relationship studies), with only low to moderate yields. Additionally, these methods face significant limitations, including long reaction times, harsh conditions, and poor overall efficiency.
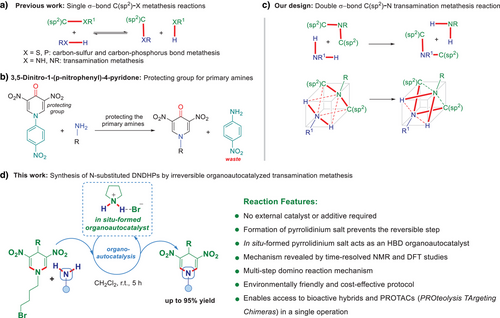
A single example for the direct interconversion of amine groups at the cyclic tertiary amine was reported for 3,5-dinitro-1-(p-nitrophenyl)-4-pyridone.[28] However, the reaction is restricted to one 1,4-dihydropyridone substrate, which was conceived and used exclusively as a protecting group for primary amines (Figure 1b). No examples for 4-unsubstituted or 4-alkyl-, 4-aryl-substituted 1,4-DHPs were reported for the reaction with amines. Furthermore, p-nitroaniline formed as a waste, and no explanation of the potential role of the applied leaving group, p-nitroaniline, was provided, and no mechanistic studies were carried out.[28] A direct and simple interconversion of functional groups at the N-atom of 4-unsubstituted and 4-alkyl-/aryl-substituted 1,4-DHPs, resulting in a broad variety of N-substituted 1,4-DHPs, which evidently could have an impact on medicinal chemistry and material science, has not been reported so far. Herein, we describe the development of a transamination metathesis of two C(sp2)─N σ-bonds (Figure 1c) in 1,4-DHPs that allows for the desired transformation to take place in a single operation under very mild reaction conditions, without external catalysts and/or additives (Figure 1d), and involves an organoautocatalyzed process,[29-31] reducing reaction steps, effort, cost, time, and waste production.
Results and Discussion
Development of Organic Base-mediated Transamination Metathesis Reaction
Initially, we discovered that the N-pentyl-substituted DNDHP derivative, undergoes double σ-bond C(sp2)─N transamination metathesis reaction with benzylamine in the presence of DIPEA (N,N-diisopropylethylamine, 2.20 equiv.) as a base and acetonitrile (MeCN) as a solvent, and after stirring the reaction mixture at 55 °C overnight. Nonetheless, we did not observe a complete conversion due to the reversibility of this transamination reaction. The highest observed isolated yield for N-benzyl-substituted DNDHP product was 52% (Figure S1). We anticipated that the introduction of a suitable substituent at the nitrogen of 1,4-DHP derivatives, e.g., bromobutyl group, rendering the secondary amine–pyrrolidine–a good leaving group, might allow performing the direct and irreversible transamination of N-bromobutyl-substituted 1,4-DHPs and primary amines (Figure 2a). To validate the feasibility of the proposed irreversible double σ-bond C(sp2)─N transamination metathesis reaction, we selected readily accessible N-bromobutyl-substituted 1,4-DHP derivatives 1a–e as substrates. We anticipated that the leaving group 4-bromobutan-1-amine would spontaneously cyclize to form pyrrolidine, a transformation that prevents the reverse reaction to the initial 1,4-DHP compounds. This inherent irreversibility was expected to improve the efficiency of the process.
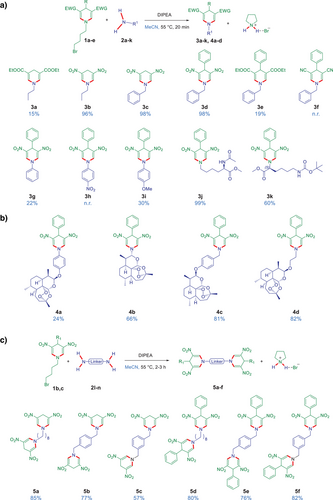
The initial transamination reactions with N-bromobutyl-substituted 1,4-DHP derivatives were carried out using 1.00 equiv. of 1,4-DHPs 1a–e, 1.10 equiv. of amines 2a–k, and 2.20 equiv. of DIPEA as a base. The reactions proceeded at an elevated temperature of 55 °C in MeCN, completing in 20 min (Figure 2a). The type of electron-withdrawing substituents at 3,5-position in 1,4-DHP has an impact on the reaction outcome. For instance, application of 3,5-diester- or 3,5-cyano-substituted 1,4-DHPs resulted in the desired products in less than 20% yield (3a, 3e, 3f), whereas their 3,5-dinitro analogues (DNDHPs) afforded the products in up to 98% yield (3b–d). Therefore, further reactions were carried out solely using DNDHPs as substrates.
Whereas the application of the amino acid L-Lys (L-lysine) with a protected side chain and an unprotected secondary amine resulted in 3k with 60% yield, the use of L-Lys with a protected secondary amine and an unprotected side chain, which functioned as a primary amine, gave the product 3j with 99% yield. Furthermore, while applying aromatic amines decreased the yield, which ranged from no reaction (n.r.) to 30% (3g–i), all primary amines gave the products with high yields. This trend persisted in synthesizing artemisinin-containing hybrid compounds (4a–d, Figure 2b). Additionally, the formation of dimeric DNDHP compounds required extended reaction times of 2–3 h, yielding dimers 5a–f in 57% to 85% (Figure 2c).
Development of Organoautocatalyzed Transamination Metathesis Reaction
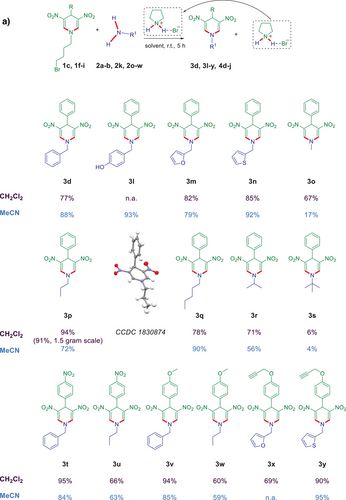
We envisioned that the in situ-formed pyrrolidine or pyrrolidinium salt could act as an organoautocatalyst for the novel transamination process. To validate this hypothesis, we conducted the reaction without adding any external catalyst or additive, testing it in two different solvents, CH₂Cl₂ (dichloromethane) and/or MeCN (Figure 3a). The reactions were carried out at room temperature for 5 h, using DNDHPs 1c, 1f–i and amines 2a–b, 2k, 2o–w. Delightfully, under these conditions, the reaction products 3d, 3l–r, and 3t–y were isolated in good to excellent yields (up to 95%). Only a single reaction towards N-tert-butyl-substituted product 3s showed merely low conversion in both solvents. A possible explanation for the low yield might be the sterical hindrance of the entering tert-butyl group of the used tertiary amine. All investigated primary and secondary amines, containing different aliphatic moieties and aromatic/heteroaromatic rings, were highly compatible, as demonstrated by the formation of 3d, 3l–y (Figure 3a). Substrates 1f–h, containing electron-withdrawing (e.g., NO2) and electron-donating (e.g., OCH3, OCH2CCH) groups in the para position of the 4-aryl substituent, were also compatible (products 3t–y, up to 95% yield). Notably, a gram scale reaction (1.5 g of 1c) in CH2Cl2 gave product 3p in 91% yield, which is only three percent less than for the small-scale experiment. The crystal structure of compound 3p was also determined (Figure 3a).
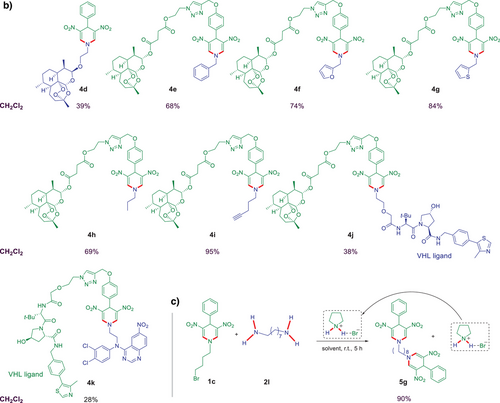
Developing new drug compounds has been a crucial aspect of modern medicine in addressing the growing public health challenges posed, e.g., by viral infections, cancer, and malaria. More recently, new drug development has also focused on well-established hybridization concepts.[32-38] Our group reported several highly efficient antiviral, anticancer, and antimalarial artemisinin-based hybrid compounds that can even overcome multidrug resistance.[39-43] Therefore, we decided to apply our novel external catalyst-free methodology to the synthesis of new artemisinin-based hybrid compounds 4d–i (Figure 3b). The reaction of 4-phenyl substituted DNDHP 1c with artemisinin-based primary amine 2k resulted in hybrid compound 4d with 39% yield. Application of DNDHP 1i, containing an artemisinin moiety at the 4-aryl substituent, and different amines 2a–b, 2p–q, 2v–w, allowed the generation of new hybrid compounds 4e–i with good to excellent yields (68%–95%, Figure 3b).
Innovative new approaches such as PROTACs (PROteolysis TArgeting Chimeras) have recently emerged in response to the existing challenges in treating cancer and viruses. A PROTAC is a bifunctional molecule designed to induce targeted protein degradation. It consists of three key components: i) Targeting ligand – a drug moiety that binds specifically to the protein of interest; ii) E3 ligase ligand – a component that recruits an E3 ubiquitin ligase; iii) Linker – a chemical spacer connecting the two ligands. By bringing the target protein and the E3 ligase into close proximity, PROTACs facilitate the ubiquitination of the target protein, marking it for degradation by the proteasome. This mechanism allows for selective protein degradation, offering a promising new mechanism of action that could potentially overcome the limitations of traditional anticancer and antiviral therapies.[44-47] We, therefore, used our new methodology for the straightforward synthesis of two novel PROTAC compounds 4j–k (Figure 3b). We applied VHL (von Hippel–Lindau), a widely expressed E3 ligase ligand, making VHL-based PROTACs applicable across various cell types.[48] Notably, an artemisinin-containing DNDHP substrate 1i, and a VHL-based primary amine were applied to synthesize PROTAC 4j. On the other hand, a VHL-containing DNDHP substrate 1j and a quinazoline-based primary amine were used to synthesize new PROTAC 4k. These results highlight the significant potential of the developed reaction to efficiently generate diverse libraries of bioactive hybrid molecules and PROTACs in a single operation. All newly synthesized hybrid compounds and PROTACs are currently undergoing biological evaluation, with detailed results to be reported elsewhere.
Notably, the novel external catalyst-free methodology also allowed the synthesis of DNDHP dimers, as exemplified in Figure 3c. The reaction of DNDHP 1c with 1,8-diaminooctane (2l) in CH2Cl2 as a solvent resulted in a dimer product 5 g with 90% yield.
Study of the Reaction Mechanism Using Nuclear Magnetic Resonance Spectroscopy and Mass Spectrometry
To investigate the mechanism of the transamination process, the reactions of DNDHP 1c and benzylamine and/or propylamine towards 3d and 3p, respectively, were monitored using time-resolved 1H-NMR and 2D HSQC (Heteronuclear Single Quantum Coherence) Nuclear Magnetic Resonance (NMR) spectroscopy (Figures S2–S15), ESI–HRMS and GC/Mass Spectrometry (MS) methods (Figures S16–S20). Compound 1c (1.0 equiv.) was mixed with benzylamine (1.0 equiv.) or propylamine (1.0 equiv.) in deuterated dichloromethane (CD2Cl2, also used as an internal standard), and 1H-NMR spectra were recorded every 3 min over 160 min. The fragments of time-resolved 1H-NMR spectra for the selected reaction of DNDHP 1c and benzylamine are presented in Figure 4a. These spectra revealed a rapid consumption of starting compound 1c and benzylamine and a fast formation of product 3d and pyrrolidine/pyrrolidinium salt. Conversion of 1c to product 3d and pyrrolidine/pyrrolidinium salt was determined to be 99% complete. Only traces of starting material 1c and benzylamine were detectable. The conversion rate was determined by comparing the integral heights of the starting material and the products. After plotting the relative concentration of products 3d and pyrrolidine/pyrrolidinium salt against the reaction time, sigmoidal curves are obtained, characteristic of autocatalytic processes (Figure 4b). From this, it can be deduced that the formation of pyrrolidine/pyrrolidinium salt promotes the formation of further product 3d as an in situ-formed organocatalyst. To confirm, we next determined reaction order with respect to pyrrolidinium salt (named “D” in the Supporting Information), by employing the kinetic method of initial rates (see Figures S21, S22 and corresponding discussion of reaction order determination using the initial rate method in Supporting information), using a double-logarithmic plot of ln(r) (for monitored reaction rate r) versus ln[D] (with pyrrolidinium salt relative concentration [D]) (see Figure S22). The slope of the linear regression line (with a goodness-of-fit R-squared value of 0.9971), 0.82, gives the reaction order of pyrrolidinium salt in the reaction of 1c with benzylamine. If pyrrolidininium salt was the only product, or, alternatively, acted as an ordinary catalyst, the reaction order would be zero.[49] By contrast, an autocatalyst also enters the left-hand side of the reaction equation, resulting in a reaction order of one for linear autocatalysis (for details, see Figures S21, S22). The actual non-integer value of the slope indicates that the process is more complex and is close to–but not exactly equivalent–to, purely linear autocatalysis, probably because of some involved pre-equilibrium.
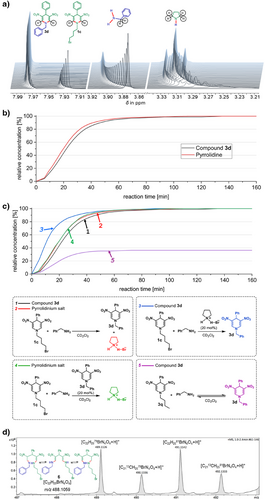
To further validate our hypothesis, we designed three test reactions (Figure 4c), which we monitored using 1H-NMR spectroscopy. The sigmoidal curves “1” and “2” illustrating the formation of DNDHP 3d (Figure 4c, black: “1”) and pyrrolidinium salt (Figure 4c, red: “2”) were used as references. In the first test reaction, the addition of pyrrolidinium salt (20 mol%) significantly accelerated the formation of DNDHP 3d (Figure 4c, blue: “3”), confirming its catalytic role in the reaction. In contrast, the addition of DNDHP 3d (20 mol%) to the reaction mixture had no discernible effect on the formation of pyrrolidinium salt (Figure 4c, green: “4”), indicating that DNDHP 3d neither catalyzes nor inhibits the transamination process. Finally, replacing N-bromobutyl-substituted DNDHP 1c with N-pentyl-substituted DNDHP 3q prevented the in situ formation of pyrrolidinium salt. As an outcome, the reaction reached an equilibrium (Figure 4c, purple: “5”), resulting in a mixture of DNDHP product 3d (36%) and unreacted DNDHP compound 3q (64%). This finding confirms that bromobutyl-substituted DNDHP 1c is essential for pyrrolidinium salt formation and subsequent reaction progression. These observations collectively underscore the pivotal role of the in situ-formed pyrrolidinium salt as an organoautocatalyst.
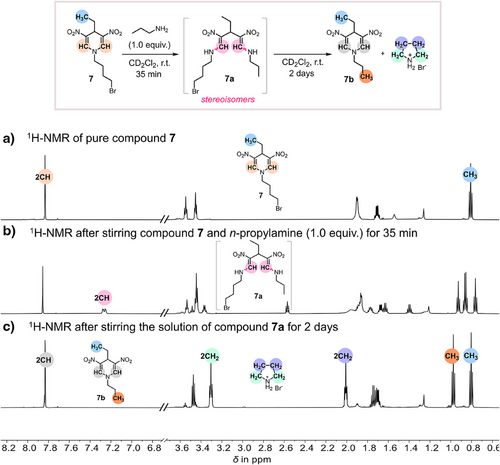
To gain further insights into the reaction mechanism, HRMS-ESI-TOF (High-Resolution Mass Spectrometry–Electrospray Ionization–Time of Flight) analysis of the reaction mixture was conducted (Figures 4d and S16). Besides the peaks for the starting materials and the product, another isotope pattern of bromide and carbon was visible in the mass spectrum, and the structure of the intermediate was assigned to ring-opened dienamine intermediate 6 and its imine-enamine tautomers. To further validate the formation of ring-opened intermediates via 1H-NMR, we selected 4-ethyl-substituted DNDHP 7 as a model compound for its reaction with n-propylamine (Figure 5). The 4-ethyl-substituted DNDHP 7 and n-propylamine were chosen to minimize signal overlap in the aromatic region, ensuring a more explicit spectral interpretation. The 1H-NMR spectrum of compound 7 in CD2Cl2 (before amine addition) is presented in Figure 5a. After adding n-propylamine (1.0 equiv.) to compound 7, followed by cooling to −20°C for 35 min, we observed the appearance of two distinct doublets at 7.26 ppm (Figure 5b). These newly emerged doublets were assigned as CH signals of enamine protons, providing evidence of a ring-opened intermediate 7a. After 2 days at room temperature, intermediate 7a underwent complete conversion into the desired transamination product 7b, accompanied by elimination of pyrrolidinium salt (Figure 5c). This observation supports the hypothesis that the reaction proceeds via a transient ring-opened intermediate before cyclization to the final transaminated product.
Next, two additional reactions were monitored by 1H-NMR (Figure 6b,c) and presented using the transformation from 7 to 7b via ring-opened intermediate 7a as a reference (Figure 6a). In the selected reaction of 4-unsubstituted DNDHP 8 with a substoichiometric amount of n-propylamine (0.7 equiv.) at room temperature, we found distinct signal sets corresponding to different acyclic dienamine's stereoisomers (Figures 6b and S23–S25). The reaction mixture, with 8a as the ring-opened intermediate, was analyzed using various correlation spectroscopy techniques (HH, CH, NH) to elucidate its structural features. Among these, the 1H,1H COSY (Proton-Proton Correlation Spectroscopy) spectrum provided the most instructive insights into the enamine region (Figure 7). The spectrum reveals the coupling of eight CH doublets with eight NH hydrogens, indicative of distinct stereoisomeric forms (EE, ZE, EZ, ZZ). Notably, four NH resonances exhibited a significant upfield shift, which we attribute to the Z-configured isomers. These hydrogen atoms experience substantial deshielding (9.3–9.8 ppm), a phenomenon likely driven by intramolecular hydrogen bonding with the adjacent nitro group. In contrast, NH hydrogens in the E-configured isomers do not engage in such hydrogen bonding and, consequently, do not exhibit similar deshielding effects. This study underscores the stereochemical complexity introduced by ring-opening and suggests that the reaction follows a dynamic equilibrium between stereoisomers due to the high flexibility of intermediate 8a. Notably, under these conditions, the four stereoisomers (EE, ZE, EZ, ZZ) of intermediate 8a remained stable. Subsequently, an additional 0.5 equiv. of n-propylamine was added. To our surprise, and in contrast to substrate 7 (Figure 6a), the stable symmetric ring-opened product 8b was formed as a mixture of three possible stereoisomers (EE, ZE, ZZ), and no transamination product was detected (see Figures 6b and S25). These findings provide strong evidence for a ring-opening transamination mechanism involving a well-defined acyclic intermediate, offering deeper mechanistic insights into the reaction dynamics.
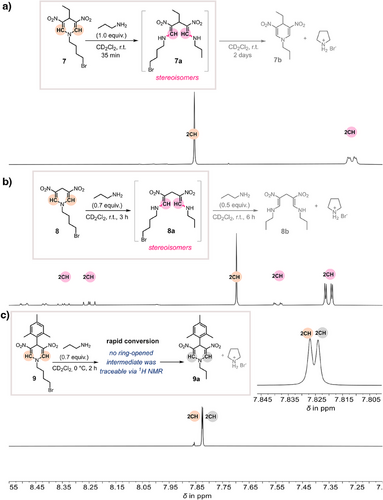
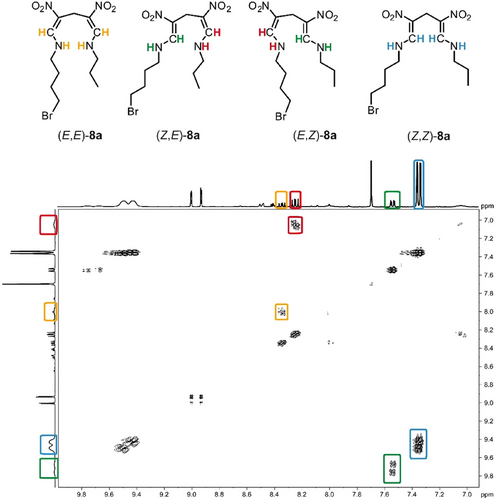
Since the substrate scope (Figure 3) and all kinetic studies (Figure 4) were conducted with 4-aryl-substituted DNDHPs, we also investigated the reaction of the 4-mesityl-substituted DNDHP compound 9 with n-propylamine (Figures 6c and S26, S27). After adding a substoichiometric amount of n-propylamine (0.7 equiv.) to the starting compound 9, the reaction mixture was cooled to 0 °C and subsequently analyzed. Unlike compounds 7 and 8, no ring-opened intermediates were observed within the 1H-NMR timescale. Instead, we detected a gradually increasing signal set corresponding to the transamination product 9a (see Figures 6c and S26, S27), suggesting a highly efficient reaction pathway that bypasses the detectable accumulation of ring-opened intermediate species. This observation implies that compound 9 undergoes a rapid transformation, potentially due to its structural features, e.g., 4-aryl substituent, favoring fast transamination without prolonged intermediate stabilization.
These findings, presented in Figure 6 provide insights into the mechanistic variations among different substrates, demonstrating that structural factors of substrates significantly influence the formation and stability of ring-opened intermediates. While 4-alky-substituted and 4-unsubstituted DNDHPs 7 and 8 exhibit clear evidence of transient acyclic dienamine species 7a and 8a, 4-aryl-substituted DNDHP 9 appears to follow a more rapid conversion to the final product. These results align with the presented substrate scope, and observed product yields underscore the role of substrate-dependent reactivity in transamination processes and also reinforce the pivotal role of pyrrolidinium salt elimination in driving the process forward.
Reaction Mechanism Based on DFT Calculations
Next, we used DFT calculations to elucidate the detailed mechanism of the developed transamination metathesis reaction. The mechanism was studied using 1-methyl-3,5-dinitro-4-phenyl-1,4-dihydropyridine (see compound 3o in Figure 3a) as a model compound, simplifying calculations and reducing conformational flexibility, while methylamine was used as a model for primary amines.
The calculations were performed with the B3LYP (Becke, 3-parameter, Lee-Yang-Parr) functional including solvation effects using the SMD (Solvation Model based on Density) model for dichloromethane. Full energy profiles and extended discussion are provided in the Computational Supporting Information (Figures S28–S32).
Based on these calculations, we propose the mechanism shown in Figure 8, which accounts for the organoautocatalysis observed in the kinetics experiments above. The mechanism involves the following sequence of reactions: 1) aza-Michael reaction, 2) deprotonation of the resulting ammonium ion by another molecule of primary amine, 3) ring-opening reaction, 4) imine-enamine tautomerization, 5) intramolecular aza-Michael reaction, 6) formation of pyrrolidinium group through an intramolecular SN2 reaction on the alkyl bromide as a rate-determining step, 7) pyrrolidine and product elimination. Notably, the protonation of the ring-opened intermediate by methylammonium bromide to form dienamine is exergonic and reversible. The dienamine could thus be an intermediate in the reaction or an off-cycle species, consistently with experimental NMR observations.
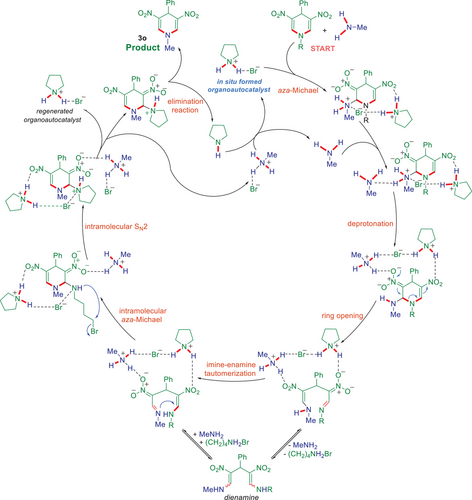
In the initial stages, the reaction proceeds only involving the DNDHP substrate and the alkylamine, with the alkylamine acting both as a reagent and as a base co-catalyst. The alkylamine base (e.g., methylamine) is regenerated after each cycle by proton transfer to the newly formed pyrrolidine, which is a stronger base. Thus, as the reaction progresses, the pyrrolidinium bromide accumulates. The calculations demonstrate that the in situ-formed pyrrolidinium bromide salt accelerates the reaction by hydrogen bonding to the nitro/nitronate and amine/ammonium groups, thereby stabilizing the charges and lowering the barriers for several steps of the multi-step domino process. This mechanism explains the experimentally observed autocatalytic rate acceleration.
Conclusion
We developed an organoautocatalyzed transamination metathesis reaction using N-bromobutyl-substituted DNDHPs as substrates. The metathesis of two C(sp2)─N bonds in these cyclic tertiary amines proceeds under exceptionally mild conditions at room temperature within only 5 h and without the use of any external catalyst or additive. Instead, in situ-formed pyrrolidinium salt acts as a hydrogen bond donor (HBD) organoautocatalyst in this process, resulting in novel DNDHPs in mostly very good to excellent yields (up to 95%). The methodology, employing a multi-step domino reaction mechanism, enables the rapid structural diversification of N-substituted DNDHPs in a single step, demonstrating excellent functional group tolerance. The straightforward synthesis of selected artemisinin-based hybrid compounds and PROTACs, which could have potential applications in treating viral infections and cancer, was also demonstrated.
Additionally, detailed studies of the reaction mechanism using time-resolved 1H-NMR, 2D NMR, and DFT methods revealed the autocatalytic nature of the process, where the in situ-formed pyrrolidinium salt facilitated the reaction progression. The formation of acyclic dienamine intermediates was observed, confirming that the reaction proceeds via ring-opened species before cyclization to the final product. The stability of these intermediates was influenced by the substituent at C-4 of the DNDHP substrate, with 4-aryl-substituted DNDHP derivatives favoring rapid transamination without accumulation of ring-opened intermediates.
We anticipate that the organoautocatalytic transamination metathesis reaction introduced here will create new attractive opportunities to synthesize new N-substituted 1,4-DHPs of interest for life and material sciences.
Acknowledgements
S.B.T. gratefully acknowledges financial support from the Deutsche Forschungsgemeinschaft (DFG, grant TS 87/28–1) and the Volkswagen Foundation (grant AZ-9B783-M. M./S. B. T.). F.H. thanks the Swedish Research Council (2019–04010) for financial support. S.S. acknowledges the CINECA award under the ISCRA initiative for the availability of high-performance computing resources and support. The authors thank Dr. F. Hampel (Friedrich-Alexander-University Erlangen-Nürnberg (FAU)) for the assistance with the X-ray diffraction analysis of compound 3p.
Open access funding enabled and organized by Projekt DEAL.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.