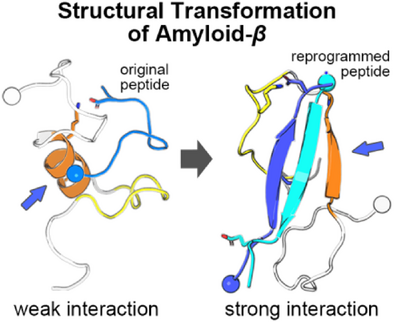
Antiparallel β-Sheet as a Key Motif of Amyloid-β Inhibitor Designed via Topological Peptide Reprogramming
Graphical Abstract
Targeting Amyloid-β (Aβ) aggregation requires a peptide-based approach that accounts for the structural heterogeneity of Aβ. Here, we employ topological reprogramming via disulfide bonds to stabilize antiparallel β-sheets within the dimeric peptide. This strategy overcomes limitations of linear peptides by creating structured complexes that resist aggregation, offering a novel tactic for tackling AD-related protein aggregation.
Abstract
Peptide inhibitor design targeting self-assembly of amyloid-β (Aβ) represents a promising strategy for suppressing the pathogenic mechanism of Alzheimer's disease (AD). Conventional approaches have primarily mimicked repetitive sequences found in fibrillar structures of Aβ aggregates. However, since the inherent flexibility of Aβ structures promotes the structural changes in the early-stage oligomerization, a structural modulation should be considered in the design of peptide inhibitors. Herein, we introduce topological reprogramming of peptides to control the structural transformation in pathogenic Aβ 1–42 (Aβ42). The eleven-residue peptide scaffold Pa11 (14HQKLVNFAEDV24) identified through the initial screening was dimerized via a disulfide bond. The dimerization stabilizes Aβ42 into higher order structures by promoting antiparallel β-sheet conformations, thereby significantly suppressing Aβ42 aggregation. Our approach underscores that modification in peptide connectivity would be a breakthrough for controlling the intrinsic flexibility of Aβ, surpassing the limitation in conventional, one-dimensional peptide building block.
Introduction
Peptides mediate physiological functions of living organisms as signaling molecules in metabolic regulation, neurotransmission, and immune response.[1-5] Recent advancements in structure-based modification strategies have significantly expanded the potential applications of peptides beyond their original biological roles.[6-10] For example, Semaglutide (Wegovy), developed by Novo Nordisk for chronic weight management, is a derivative of the hormone glucagon-like peptide-1 (GLP-1).[11, 12] The modification of GLP-1 substantially increases the peptide's half-life by enhancing its binding affinity to serum albumin (conjugation of fatty acid) and replacing vulnerable positions for enzymatic hydrolysis (replacement to α-aminoisobutyric acid).[11] This example underscores the significance of rational modification in peptide structure to address challenges in biological regulation or disease-related phenomena as proposed in multiple examples.[6, 11, 13-21] Hence, modification based on the molecular understanding of peptide structure could be a novel approach for overcoming limitations of conventional peptides.
Alzheimer's disease (AD), a leading cause of dementia, is associated with the pathogenic aggregation of amyloid-β (Aβ) peptides. Aβ peptides are generated through the sequential enzymatic cleavage of amyloid precursor protein (APP).[22, 23] The cleaved Aβ peptides with hydrophobic sequence self-assemble to highly ordered, β-sheet rich fibrillar aggregates that initiate the pathological cascade of AD in the human brain.[24] Peptide-based inhibitors of amyloid aggregation represent a well-established strategy for suppressing Aβ aggregation or eliminating preexisting aggregates.[25-38] The fundamental concept underlying these inhibitors is rooted in the amyloid peptide sequence in the repeating β-sheet spine of fibrillar aggregates. While monomeric Aβ structures are conformationally flexible, their hydrophobic sequences specifically recognize identical counterparts of the others to form fibrillar aggregates, as observed in atomic-resolution PDB structures.[39-41] Inhibitor design utilizes such intermolecular interactions by mimicking Aβ sequences often with incorporating chemical modifications—such as cyclization,[30, 42] mutation,[25, 35] or conjugation[33]—to prevent further aggregation of Aβ-inhibitor complexes. Molecular recognition via the β-sheet repeating unit offers a straightforward and highly efficient approach to designing peptide-based inhibitors. However, the mechanism of action for these inhibitors remains elusive due to the structural heterogeneity of Aβ peptides in the early stages of aggregation. Still, modifying peptide inhibitors may present a novel avenue for advancing amyloid aggregation inhibitors, although a rational design approach is challenging without a thorough understanding of the structural dynamics of Aβ.
Our previous studies highlighted two critical factors for reducing the aggregation propensity of Aβ: (i) specific point mutations within the inhibitor and (ii) the design of inhibitor sequences derived from the native sequence of the Aβ peptide.[35, 43] The current study newly proposes that topological modification of peptide can generate structural motif that significantly improves the inhibitory action in Aβ aggregation. Our strategy employs computational screening of peptide-peptide interactions that induce the formation of structurally ordered conformers from intrinsically disordered structures of Aβ and its inhibitor. Computationally screened, one-dimensional peptide Pa11 (HQKLVNFAEDV) is the optimal candidate for suppressing Aβ42 aggregation. Using this optimized scaffold, we have induced the dimerization of Pa11 peptide through disulfide bond formation. The dimerization induced antiparallel β-sheet conformation of Pa11 scaffolds. The enhanced structural rigidity of the Pa11 dimer promotes complexation with Aβ42 and ultimately its inhibitory action during the aggregation. This approach, in turn, significantly reduced aggregate-mediated cytotoxicity to SH-SY5Y cells.
Overall, we have demonstrated that the structural motif of the dimerized Pa11 inhibitor plays an essential role in the suppression of Aβ42 aggregation. Given that traditional peptide inhibitors are structurally disordered, our observation emphasizes that higher order structures of peptide inhibitors are potentially crucial as the indispensable component of peptide inhibitors.
Results and Discussion
Theoretical Screening of Peptide Scaffold
The primary driving force behind the aggregation of intrinsically disordered Aβ42 conformers to form fibrils is the hydrophobic clustering of nonpolar residues.[44, 45] Therefore, disruption of hydrophobic clustering is critical for inhibiting the early oligomerization stages of Aβ42. Secondary structural predictions of Aβ42, obtained through the s2D method, reveal that the central region (K16–A21) and the C-terminal region (G38–A42) inherently favor β-sheet formation (Figure 1a).[46] Given the crucial role of β-sheet structures in aberrant protein assembly, region-specific targeting of these segments could be a promising strategy to disrupt Aβ42 aggregate formation. To test this hypothesis, we employed peptide inhibitor candidates—Pa and Pb, listed in Table S1—that were previously designed based on the two cross-β regions in the fibrillar structure of Aβ42.[35] We performed replica exchange molecular dynamics (REMD) simulations (detailed in the Methods section) to characterize the molecular behavior of these peptide candidates in the presence of Aβ42 and to assess their impact on the structural dynamics of Aβ42 (Figures S1–S3). We systematically varied their lengths from five to thirteen amino acids to explore the influence of one-dimensional extension of the peptide length on molecular interactions (Figure 1b).
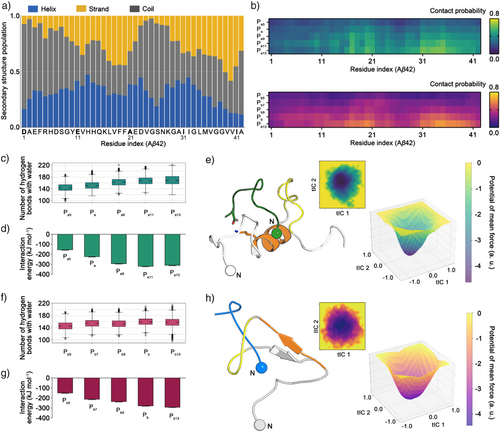
The interactions between Pa-derived peptides and Aβ42 are predominantly concentrated in the central region (K16–A21) and the C-terminal region (A30–V36). The contact probability (detailed in the Methods section) between Aβ42 and the inhibitors increases with peptide length. Notably, the contribution of the C-terminal region of Aβ42 in the contact probability gradually increases from Pa5 to Pa13 (Figures 1b and S4). Our simulations revealed that the positively charged N-terminus and lysine residues of Pa-derived peptides interact with the negatively charged Asp7, Glu11, Glu22, or Asp23 residues of Aβ42 (Figures S4 and S5). Conversely, the C-terminus and negatively charged residues (Asp, Glu) of Pa-derived peptides form interactions with the N-terminus, Lys16, or Lys28 of Aβ42. Additionally, the nonpolar side chains of Aβ42, particularly residues 17LVFF20, exhibit limited exposure to water molecules, favoring intermolecular interactions with Pa peptides. The longest Pa-derived peptide, Pa13, forms a salt bridge between its N-terminus and the C-terminus of Aβ42, while its C-terminus interacts with Lys28 of Aβ42, stabilizing the complex differently from the Pa11 (Figure S4). Overall, the arrangement of charged and nonpolar residues in Pa-derived peptides promotes frequent contacts with the central region of Aβ42, a trend that becomes more prominent with increasing peptide length. Similarly, the interaction map of Pb-derived peptides shows a gradual increase in contact probability with the central region (K16–A21) and the C-terminal region of Aβ42 as peptide length increases (Figure 1b).
To investigate the structural rearrangement of Aβ42 induced by the designed peptides, we analyzed the number of hydrogen bonds, the theoretical interaction energy, and the free energy landscape concerning for the dihedral angles of Aβ42 using the REMD simulation trajectories (Figure 1c–h). The number of hydrogen bonds between water molecules and Aβ42–peptide complexes gradually increases but plateaus with Pa11 and Pa13.
This trend suggests that the displacement of water molecules from the hydrophobic regions of Aβ42 into the bulk solvent, indicative of enhanced hydrophobic interactions, is maximized at Pa11 and Pa13 (Figure 1c).[47] Similarly, the interaction energy between Aβ42 and each peptide levels off with Pa11 and Pa13, indicating that increasing peptide length beyond eleven residues does not contribute to stronger interactions with Aβ42 (Figure 1d). The representative structure of Aβ42 in complex with Pa11 reveals that Pa11 binds to the central region of Aβ42, effectively shielding the critical oligomerization domain responsible for aggregation (Figure 1e).[35, 48] In the case of Pb-derived peptides, a higher degree of interactions is observed in the longer peptide (Pb9, Pb, and Pb13) (Figure 1f,g). However, the conformational landscape of Aβ42 is perturbed differently by Pb-derived peptides (Figures 1h and S6). When complexed with Pb13, Aβ42 continues to adopt partially folded conformations where the central region interacts with the C-terminal region (Figure 1h).
Although both Pa- and Pb-derived peptides interact with Aβ42, distinct interaction patterns were observed in the structural dynamics of Aβ42. Also, the molecular interaction with C-terminal region of Aβ42 is enhanced as the peptide length increases. These insights from computational screening were examined experimentally using the peptide inhibitor candidates in Table S1.
Experimental Validation of Optimal Inhibitor Scaffold
To evaluate the effects of the inhibitor candidates, we monitored the formation of Aβ42 fibrillar aggregates using a thioflavin T (ThT) fluorescence assay (Figure 2a–e). The normalized fluorescence intensity at the endpoint (24 h) of Pa5 treated Aβ42 showed a fluorescence intensity (105.6 ± 1.8%) comparable to the control group (Aβ42 w/o inhibitor). However, the relative fluorescence intensity at the endpoint decreased to 86.1 ± 5.9% and 85.1 ± 9.6% when treated with Pa and Pa9, respectively. A more significant reduction in fluorescence intensity was observed with Pa11 (71.4 ± 4.7%), whereas the inhibitory effect of Pa13 was not significantly improved (78.3 ± 1.6%). Following the ThT assay, transmission electron microscope (TEM) images after negative staining showed a gradual shift in the length distribution of Aβ42 fibrils (Figures 2f–g and S7). The control group formed short fibrillar aggregates after the 24 h of incubation (279.4 ± 19.0 nm). In contrast, all Pa-derived peptides induced broadly dispersed, longer fibrils, especially for Pa11 and Pa13 treated Aβ42 samples (816.9 ± 44.8 nm and 784.1 ± 48.9 nm, respectively). These results suggest that Pa-derived peptides impede nuclei formation during the early stages of Aβ42 fibrillar aggregation, thereby reducing the number of nuclei available for elongation. Consequently, this mechanism results in the formation of longer fibrils with a lower number density at the endpoint.[49]
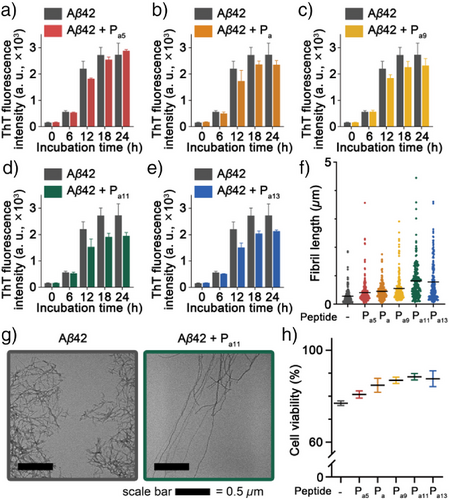
To assess the cytotoxicity of Aβ42 fibrils altered by the treatment of Pa-derived peptide, we assessed viability changes of the SH-SY5Y neuroblastoma cells (Figure 2h). Aβ42 fibrils reduced cell viability to 76.9 ± 1.0%, as measured by the modified thiazolyl blue tetrazolium bromide (MTT) assay. However, the Aβ42 fibrils co-incubated with Pa-derived peptides alleviated the cytotoxicity, and the cell viability increased up to 88.4 ± 1.4% in Aβ42 sample co-incubated with Pa11 peptide. This reduction in cytotoxicity is attributed to the inhibitory effects of Pa-derived peptides that decrease the number density of fibrillar aggregates and limit the formation of cytotoxic shorter aggregates. Consistent with MD simulations, Pa11 demonstrated the highest performance in both ThT and MTT assays. We hypothesize that the interaction energies observed in MD simulations correlate with the actual binding affinity between Aβ42 and Pa peptides, thereby influencing the suppression of Aβ42 aggregation.
To further validate this hypothesis, we examined the relative intrinsic binding affinities between Aβ42 and Pa peptides using electrospray ionization tandem mass spectrometry (ESI-MS/MS) (Figures S8, and S9). The results revealed a clear upward trend in binding affinity from Pa5 to Pa11, with a slight decline observed at Pa13. Notably, these findings are in good agreement with the trend observed in the interaction energies of MD simulations (Figure 1d). Considering that Pa11 exhibited the highest inhibitory effect among the Pa-derived peptides, we conclude that the strong association with Aβ42 is a critical factor in effectively suppressing Aβ42 aggregation and mitigating the cytotoxicity of Aβ42 aggregates. Following the analysis of the Pa-derived peptides, we investigated the effects of Pb-derived peptides on fibrillar aggregation of Aβ42. The tested Pb-derived peptides exhibited negligible effects on Aβ42 aggregation (Figure S10). The normalized ThT fluorescence after 24 h of incubation showed only a slight reduction of up to 6.4% in the Pb13-treated Aβ42 sample (Figure S10a). Also, Pb-derived peptides did not induce significant changes in the length distribution of Aβ42 fibrils (Figures S10b and S11). Consistent with these findings, the cytotoxicity of Aβ42 fibrils remained unchanged in the presence of Pb-derived peptides. This lack of reduction in cytotoxicity is attributed to the unaltered quantity and morphology of fibrillar aggregates (Figure S10c). Although MD simulations resulted in the length-dependent increase of the Aβ42-peptide contacts near the C-terminal region of Aβ42, this was not reflected in significant improvements for ThT or MTT assay results, regardless of the Pb-derived peptide length. These results are in line with our experimental data, which show the length dependent increase in the CID binding energy of Pb-derived peptide is not directly related to the suppression of Aβ42 fibrillar aggregation (Figures S10d, S12, and S13). Additionally, we confirmed that the N-terminal fragment (1DAEFRHDSGYEVH13, PN) of Aβ42 does not show any suppression effect on the Aβ42 fibrillar aggregation (Figure S14).
As discussed above, the fundamental difference between Pa and Pb is their modes of molecular interaction. The interaction mechanism of Pb-derived peptides is predominantly driven by hydrophobic interactions. By contrast, the contact mechanism of Pa peptide involves in the orchestration of electrostatic interactions by salt bridges and hydrophobic interaction facilitated by water release. Given that the early oligomerization stage of Aβ42 is primarily driven by hydrophobic interactions, a peptide inhibitor that relies solely on hydrophobicity for complex formation should be less competitive in disrupting aggregation. In this context, the eleven-residue peptide Pa11 emerges as the optimal candidate for suppressing Aβ42 aggregation. Its ability to shield the critical oligomerization domain of Aβ42 by specifically targeting the central region enhances its inhibitory efficacy.
Antiparallel β-sheet Motif of Dimerized Scaffold via Disulfide Bond Formation
By theoretical and experimental screening of peptide length and sequence, Pa11 was identified as the best candidate with the highest inhibitory performance among Pa, Pb, and PN candidates. While linear extension of peptide length improves inhibitory effects, single-chain forms (AA) can still suffer from structural fluctuations and thus face higher entropic barriers, making it difficult to achieve the desired rigid structure for effective Aβ42 interaction (Figure 3a). Therefore, we sought to further enhance the inhibitory potential of Pa11 through topological modification. Modifying structural connectivity through disulfide bond formation (e.g., Terlipressin,[50] DOTATOC,[51] Pramlintide[6]), or cyclization (e.g., Pasireotide[43] and Bremelanotide[52]) is well-known strategies to enhance molecular recognition in biologically active peptides. Covalent constraints imposed by these modifications restrict the conformational flexibility of peptides, favoring structural arrangements optimal for binding to target proteins.
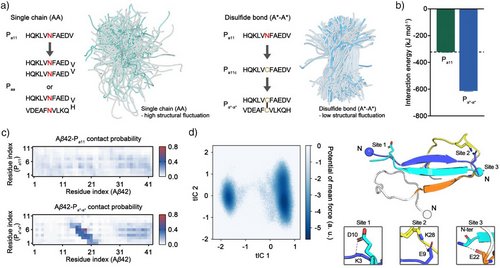
Considering the predicted secondary structure populations of Aβ42 and its fibrillar form, the regions interacting with Pa11 predominantly prefers β-sheet formation (Figure 1a).[39, 40] However, our MD simulations display that Pa11 facilitates transient helical conformations of residues 16–22, whereas random coil conformations remain dominant in the remaining residues (Figure S6). Due to these observations, we hypothesized that although Pa11 exhibits molecular recognition functionality, its preferential recruitment of the transient helical conformation may be suboptimal; therefore, stabilizing Pa11 in a β-sheet-enriched conformation could be more efficient to enhance its interaction with Aβ42. A disulfide bond is the common stabilizer of antiparallel β-sheet orientations by constraining bond lengths and supporting the hydrogen bond network of antiparallel β-sheet structures.[53-55] Given the β-sheet propensity of the Pa11 sequence, we anticipated that disulfide-bond design may promote the formation of a stabilized antiparallel orientation of the Pa11 dimer that enables multivalent interaction with a single Aβ42 molecule (Figure 3a). Consequently, we implemented a disulfide bond-mediated dimerization strategy (A*-A*) to stabilize Pa11 in the orientation necessary for enhanced inhibitory potency. To examine this idea, we theoretically generated the dimeric peptide Pa*-a* by substituting the asparagine residue in the center of Pa11 to cysteine.
To evaluate the interaction between the dimeric Pa*-a* peptide and Aβ42, we conducted REMD simulations. The theoretical interaction energy of the Aβ42–Pa*-a* complex nearly doubled compared with that of the Aβ42–Pa11 complex (−613.51 and -322.25 kJ mol−1 for Pa*-a* and Pa11, respectively) (Figures 1d and 3b). This substantial increase in binding energy suggests that the dimeric Pa*-a* forms a more stable complex with Aβ42 through multivalent interactions. The degree of molecular contact between Pa*-a* and Aβ42 is also significantly higher than that observed for Pa11 (Figure 3c). Contact probability analysis revealed that Pa*-a* preferentially interacts with the central region of Aβ42, further supporting its enhanced binding capability (Figure 3c). Furthermore, when interacting with Aβ42, the two units of Pa*-a* adopt an antiparallel orientation, with covalent constraints and interstrand salt bridges between N-termini and C-termini that further stabilize the dimerization. Each unit of Pa*-a* contains two positively charged residues (the N-terminus and a lysine) and three negatively charged residues (the C-terminus, a glutamic acid, and an aspartic acid), enabling simultaneous interstrand salt-bridge formation (within Pa*-a*) and additional salt-bridge formation with Aβ42. For example, of the two Antiparallel strands, the N-terminus of one strand forms a salt bridge with Glu22 or Asp23 of Aβ42, while the other unit binds to the C-terminal region (or end) of Aβ42 (Figures 3d, and S15). This relatively higher probability of salt-bridge formation, compared with Pa11, likely underlies the enhanced selectivity of the dimeric peptide for the central region of Aβ42 (Figures 3c, S5, and S15). Potential energy surface analysis of the Aβ42–Pa*-a* complex revealed two predominant binding modes. A deep free energy well on the lower right of the energy surface corresponds to simultaneous binding of Pa*-a* to both the central and C-terminal hydrophobic regions of Aβ42 (Figure 3d). A secondary, less populated binding mode on the lower left of the energy surface involves Pa*-a* binding primarily to the C-terminus with contacts to the central region of Aβ42 (Figures 3d and S16). Collectively, these molecular dynamics simulation results suggest that Pa*-a* not only enhances recognition of the central region of Aβ42 but also establishes stronger, multivalent interactions, effectively shielding the key oligomerization domain to potentially inhibit Aβ42 aggregation.[35, 48]
Suppression of Aβ42 Aggregation by Pa*-a* Inhibitor
We investigated the suppression of Aβ42 aggregation by the disulfide bond-linked Pa*-a* dimer. To quantify the β-sheet contents at the endpoint (24 h) of Aβ42 aggregation, we analyzed the secondary structural features of the Aβ42 in the absence or presence of Pa*-a* using circular dichroism (CD) spectroscopy (Figure 4a). The control group, consisting of intact Aβ42, showed the formation of β-sheet rich fibrillar structure. By contrast, a progressive decrease in the β-sheet content was observed with increasing Pa*-a* concentration. Notably, at a 10-fold excess of Pa*-a*, the feature of a random coil structure was dominant in the CD spectrum, indicating significant disruption of β-sheet formation (Figure 4a). This trend was observed consistently in the ThT fluorescence assay and TEM analysis after 24 h of incubation.
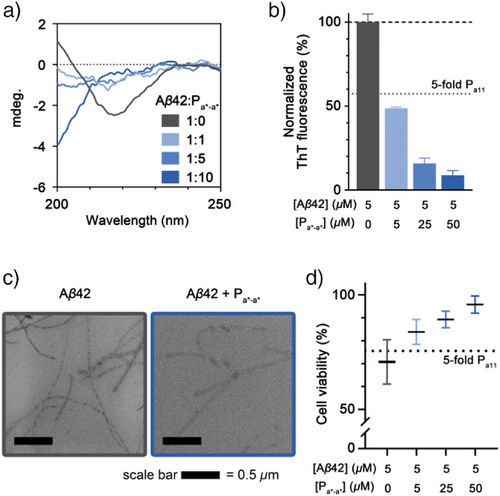
The aggregation of Aβ42 was markedly suppressed in the presence of Pa*-a* (Figure 4b,c). At an equimolar concentration of Pa*-a*, the ThT fluorescence intensity decreased to 48.7% relative to the control Aβ42 group. It is noteworthy that Pa*-a* added Aβ42 at an equimolar ratio showed lower ThT fluorescence intensity compared to the 5-fold excess of Pa11 added Aβ42 (Figures 4b, and S19). This implies that the inhibitory effect of the peptide is significantly enhanced by disulfide linked dimer. Further, reductions in ThT fluorescence intensity were observed with 5- and 10-fold excesses of Pa*-a*, decreasing to 15.7% and 8.8%, respectively (Figure 4b). TEM image analysis corroborated these findings, showing a significant reduction in fibril abundance in Pa*-a*-treated Aβ42 samples, without evidence of alternative aggregate formation (Figure 4c). Importantly, the reduction of Aβ42 aggregates led to the significant alleviation of cytotoxicity. The MTT assay demonstrated that cell viability increased from 70.7% in the control group to 95.8% in the presence of a 10-fold excess of Pa*-a* (Figure 4d), and Pa*-a* did not exhibit cytotoxicity itself (Figure S22). Moreover, Pa*-a* addition not only reduced toxicity of Aβ42 aggregates obtained at the endpoint (24 h incubation) but also mitigated the cytotoxicity of various toxic oligomeric species (Figure S23). These results collectively highlight the potent inhibitory effect of the disulfide bond-linked Pa*-a* dimer on Aβ42 aggregation and its ability to mitigate aggregate-induced cytotoxicity.
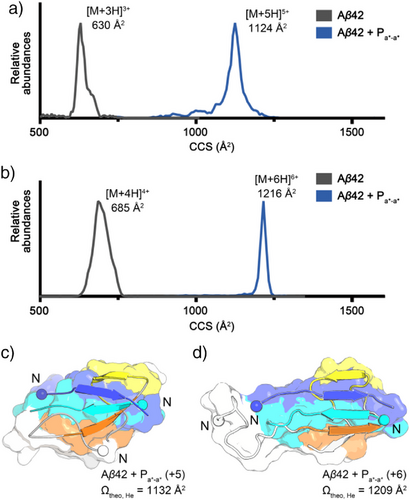
We anticipated that the binding mode of Pa*-a* with Aβ42 would lead to compelling results in ThT and MTT assays. Thus, the molecular interaction of Pa*-a* with Aβ42 was investigated using ion mobility-mass spectrometry (IM-MS) (Figure 5a,b).[56-59] The conformation of gaseous ion of the Aβ42 and Pa*-a* complex showed 1124 Å2 and 1216 Å2 of collision cross sections (CCSs) in the +5 and +6 charge states, respectively. Experimental CCS values of the complex were compared with theoretical values of in vacuo MD simulated conformations (see details in Methods section) (Figure 5c,d). The representative MD-simulated structures in solution from the free energy landscape were employed as a structural pool for modeling the gas-phase conformations (Figures 3d and S26). One hundred MD-simulated structures originated from the more stable, abundant population in solution MD simulations show a broad distribution of theoretical CCS values ranging from 1070 to 1351 Å2 (Figures S26a and S26b). It is noteworthy that this distribution includes a subpopulation centered around 1203 Å2, closely matching the experimental CCS of 1216 Å2 in the +6 charge state. If conformations of Aβ42 and Pa*-a* complex are stabilized with five or six extra charge states in the gas phase, the conformations merge to the experimental CCS values observed in +5 or +6 charge states. By contrast, MD-simulated structures of the less stable, abundant conformers show a narrow distribution of theoretical CCS values (Figure S26c) ranging from 1078 to 1243 Å2 that does not sample the ∼1216 Å2 region, indicating these conformers contribute less to the observed gas-phase structure. Therefore, we anticipate that the minor conformers in Figure 3d may not play a significant role in the suppression of Aβ42 aggregation. Furthermore, Fourier transform infrared (FT-IR) spectroscopy analysis of Aβ42 and Pa*-a* mixture exhibited a characteristic shoulder corresponding to the conventional antiparallel β-sheet conformation (Figure S28). Consequently, the IM-MS and FT-IR results strongly support the conclusion that Pa*-a* stabilizes Aβ42 through multivalent interactions as intended in our disulfide bond design, thereby suppressing the aggregation pathway (Figures 3d and 5c).
To understand the role of Pa*-a* after the initial stage, we measured relative abundances of Aβ42 oligomers and Pa*-a* complexes with monomeric or small oligomeric Aβ42 using ESI-MS (Figure S29). We found that, as the concentrations of Pa*-a* increased, the relative abundance of Aβ42 oligomers formed within 1 h decreased in a concentration-dependent manner. Moreover, MS spectra revealed clear single or multiple binding of Pa*-a* with monomeric or small oligomeric Aβ42, indicating that Pa*-a* contributes to the sequestration of Aβ42 species in the early stage. Next, we treated Pa*-a* to preincubated Aβ42 for 1, 3, or 6 h (Figure S30). We observed a decline in the inhibitory effect as the preincubation time increased, confirming that Pa*-a* acts predominantly at the early stage of Aβ42 aggregation. Finally, we tested seed-dependent aggregation in the presence of 0.01% (w/w) preformed fibrils (Figure S31). While monomeric Pa-derived peptides were not effective, Pa*-a* suppressed Aβ42 aggregation in a concentration-dependent manner. However, its inhibitory effect disappeared at higher seed ratio (0.1% w/w).
To further characterize chemical properties of Pa*-a*, We measured its solubility in buffer and stability in human plasma to assess chemical properties of Pa*-a*. Turbidity (OD600) assay in 20 mM Tris-HCl (pH 7.4) showed that Pa*-a* remained fully soluble up to 0.5 mM (1.29 mg mL−1), whereas Pa11 began to show a noticeable increase in OD600 (∼0.02) at 1 mM (1.30 mg mL−1; Figure S32). In human plasma, Pa*-a* exhibited an apparent half-life of 7.7 min, while it was 57.9 min for Pa11 (Figure S33). MS binding studies indicate that both peptides associate with human serum albumin (Figure S34), which is likely protected from proteolytic degradation. Additionally, in a confluent calf pulmonary artery endothelial cell (CPAE) monolayer transwell assay, Pa*-a* achieved 35% translocation of the donor load at 48 h, whereas no flux was observed in a parallel artificial membrane permeability assay (PAMPA) blood-brain barrier (BBB) kit (Figure S35). These data demonstrate Pa*-a* has a potential cell-mediated transportation mechanism, rather than a simple diffusion across the lipid membrane.
Conclusion
In this study, we propose a peptide design strategy that combines computational screening of molecular recognition and covalent modification via disulfide bond linkage for developing a high-performance inhibitor of Aβ42 aggregation. The final output, the dimeric Pa*-a* peptide linked via a disulfide bond showed significant improvements in suppressing Aβ42 aggregation compared to its monomeric form Pa11. This enhanced inhibition effectively reduced the formation of cytotoxic Aβ42 species. Notably, the inhibitory effect of Pa*-a* is superior not only to the monomeric Pa-derived peptides but also to one of the traditional β-sheet breakers such as LPFFD (Figure S36). This observation further emphasizes the advantage of our covalent dimerization strategy. Overall, our results highlight that the disulfide bond linkage of monomeric peptides is a straightforward modification for boosting molecular recognition of amyloid protein motifs, particularly those with high β-sheet propensity. While peptide modification using the disulfide bond is frequent in therapeutic peptides, its potential for amyloid aggregation inhibitors has not yet been fully explored. Hence, this approach could be expandable for broader applications in targeting diverse aggregation-prone proteins, such as tau, α-synuclein, TDP-43, and FUS, which are associated with various neurodegenerative diseases.
Although the flexible nature of amyloidogenic intrinsically disordered proteins (IDPs) makes them susceptible to aberrant protein self-assembly, their structural plasticity typically assists molecular interactions with diverse biological partners. However, due to technical limitations, targeting disordered regions of IDPs as recognition motifs for functional modulation remain a significant challenge. This limitation complicates efforts to precisely control functions of IDPs. A key concept in our current simulation-driven peptide design is the potential controllability of complex formation between two intrinsically disordered species. Conformational switching from α-helix to β-sheet in local structures of Aβ42 is a good example to demonstrate that even IDPs can be guided to adopt defined structural features by design. Even in nature, it is rare for two intrinsically disordered proteins to form well-ordered complexes. Therefore, our research holds promise as a critical milestone in developing strategies to recognize and structurally organize specific motifs within IDPs.
Supporting Information
The authors have cited additional references within the Supporting Information.[60-76]
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Science and ICT) (RS-2023–00213155 and RS-2023–00221182 to T.S.C. & RS-2025–00523380 to H.I.K. & RS-2023–00274504 to D.I.), Korea Basic Science Institute (KBSI) National Research Facilities & Equipment Center (NFEC) funded by the Korea government (Ministry of Education) (RS-2024–00402934 to T.S.C. and 2019R1A6C1010028 to T.S.C. and H.I.K.), Korea Health Industry Development Institute (KHIDI) and Korea Dementia Research Center (KDRC) funded by the Korea government (Ministry of Health & Welfare, Ministry of Science and ICT) (RS-2024–00332875 to H.I.K.) and grants from the National Institutes of Health (NIH) (R01HL155532 and R35HL150807 to W.A.G.). This study was supported by a grant from the Yuhan Innovation Program (YIP) of Yuhan Corporation. This work was also supported by the National Institute of Supercomputing and Network/Korea Institute of Science and Technology Information with supercomputing resources including technical support (KSC-2023-CRE-0541 and KSC-2024-CRE-0104). The authors thank the National Center for Seoul National University Research Facilities for their assistance with TEM measurements and the Institute for Basic Science (IBS) Center for Molecular Spectroscopy and Dynamics (IBS-R023-D1) for providing JASCO J-815 circular dichroism spectrophotometer time and professional technical support.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.