Hierarchically Self-Assembled Anion-Coordination-Driven Gels for Guest Segregation and Electrical Sensing
This article relates to:
-
Inside Front Cover: Hierarchically Self-Assembled Anion-Coordination-Driven Gels for Guest Segregation and Electrical Sensing (Angew. Chem. Int. Ed. 28/2025)
- Volume 64Issue 28Angewandte Chemie International Edition
- First Published online: June 2, 2025
Jie Zhao
Key Laboratory of Synthetic and Natural Functional Molecule of the Ministry of Education, College of Chemistry and Materials Science, Northwest University, Xi'an, 710069 China
School of Chemistry and Chemical Engineering, Xi'an University of Architecture and Technology, Xi'an, 710055 China
These authors contributed equally to this work.
Search for more papers by this authorYidan Li
Key Laboratory of Synthetic and Natural Functional Molecule of the Ministry of Education, College of Chemistry and Materials Science, Northwest University, Xi'an, 710069 China
These authors contributed equally to this work.
Search for more papers by this authorHuidan Zhang
Key Laboratory of Synthetic and Natural Functional Molecule of the Ministry of Education, College of Chemistry and Materials Science, Northwest University, Xi'an, 710069 China
These authors contributed equally to this work.
Search for more papers by this authorRuying Lv
Key Laboratory of Synthetic and Natural Functional Molecule of the Ministry of Education, College of Chemistry and Materials Science, Northwest University, Xi'an, 710069 China
Search for more papers by this authorLe Yu
Key Laboratory of Synthetic and Natural Functional Molecule of the Ministry of Education, College of Chemistry and Materials Science, Northwest University, Xi'an, 710069 China
Search for more papers by this authorSilvia Marchesan
Department of Chemical & Pharmaceutical Sciences, University of Trieste, Via L. Giorgieri 1, Trieste, 34127 Italy
Search for more papers by this authorCorresponding Author
Dong Yang
Key Laboratory of Synthetic and Natural Functional Molecule of the Ministry of Education, College of Chemistry and Materials Science, Northwest University, Xi'an, 710069 China
E-mail: [email protected]
Search for more papers by this authorJie Zhao
Key Laboratory of Synthetic and Natural Functional Molecule of the Ministry of Education, College of Chemistry and Materials Science, Northwest University, Xi'an, 710069 China
School of Chemistry and Chemical Engineering, Xi'an University of Architecture and Technology, Xi'an, 710055 China
These authors contributed equally to this work.
Search for more papers by this authorYidan Li
Key Laboratory of Synthetic and Natural Functional Molecule of the Ministry of Education, College of Chemistry and Materials Science, Northwest University, Xi'an, 710069 China
These authors contributed equally to this work.
Search for more papers by this authorHuidan Zhang
Key Laboratory of Synthetic and Natural Functional Molecule of the Ministry of Education, College of Chemistry and Materials Science, Northwest University, Xi'an, 710069 China
These authors contributed equally to this work.
Search for more papers by this authorRuying Lv
Key Laboratory of Synthetic and Natural Functional Molecule of the Ministry of Education, College of Chemistry and Materials Science, Northwest University, Xi'an, 710069 China
Search for more papers by this authorLe Yu
Key Laboratory of Synthetic and Natural Functional Molecule of the Ministry of Education, College of Chemistry and Materials Science, Northwest University, Xi'an, 710069 China
Search for more papers by this authorSilvia Marchesan
Department of Chemical & Pharmaceutical Sciences, University of Trieste, Via L. Giorgieri 1, Trieste, 34127 Italy
Search for more papers by this authorCorresponding Author
Dong Yang
Key Laboratory of Synthetic and Natural Functional Molecule of the Ministry of Education, College of Chemistry and Materials Science, Northwest University, Xi'an, 710069 China
E-mail: [email protected]
Search for more papers by this authorGraphical Abstract
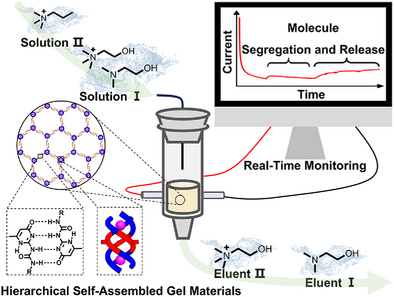
A class of gels formed through hierarchical self-assembly of “aniono” helicates were developed that can be utilized as filling material in columns and showed the ability to selectively encapsulate and release choline. The host-guest properties could also be monitored in real time by electrical characterization. This study introduces the concept of flowing a solution containing guests through a host-containing gel for effective guest separation.
Abstract
The search for macroscopic materials with differentially addressable spaces based on molecular containers has long been driven by their great potential for diverse practical applications pertaining guest selective capture and release. Herein, we report a class of gels (G1-NH2, G1-UPy, G2-NH2, and G2-UPy) that are formed through hierarchical self-assembly of anion-coordinated architectures. The well-defined shapes and sizes of the cavities within anion-coordinated architectures endow the gels with different internal phases, which is beneficial to differentiate the release profiles of guest molecules (dimethylethanolamine, choline, and propyl trimethylammonium) depending on their binding affinity. This study also introduces the concept of flowing a solution containing guests through a host-containing gel for effective guest separation. The process of guest binding could be also monitored in real time via electrical characterization. This work may thus provide a useful strategy for selective solid-phase molecular separation.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
| Filename | Description |
|---|---|
| anie202504207-sup-0001-SuppMat.pdf81.8 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1S. Juul, F. Iacovelli, M. Falconi, S. L. Kragh, B. Christensen, R. Froehlich, O. Franch, E. L. Kristoffersen, M. Stougaard, K. W. Leong, Y.-P. Ho, E. S. Soerensen, V. Birkedal, A. Desideri, B. R. Knudsen, ACS Nano 2013, 7, 9724–9734.
- 2N. Ahmad, H. A. Younus, A. H. Chughtai, F. Verpoort, Chem. Soc. Rev. 2015, 44, 9–25.
- 3E. G. Percástegui, T. K. Ronson, J. R. Nitschke, Chem. Rev. 2020, 120, 13480–13544.
- 4T. Douglas, M. Young, Nature 1998, 393, 152–155.
- 5J.-W. Yoo, D. J. Irvine, D. E. Discher, S. Mitragotri, Nat. Rev. Drug. Disc. 2011, 10, 521–535.
- 6Z. Majeed, N. K. Ramli, N. Mansor, Z. Man, Rev. Chem. Eng. 2015, 31, 69–95.
- 7K. E. Uhrich, S. M. Cannizzaro, R. S. Langer, K. M. Shakesheff, Chem. Rev. 1999, 99, 3181–3198.
- 8X. Huang, C. S. Brazel, J. Controlled Release 2001, 73, 121–136.
- 9C.-C. Lin, K. S. Anseth, Pharm. Res. 2009, 26, 631–643.
- 10A. Herrmann, Angew. Chem. Int. Ed. 2007, 46, 5836–5863.
- 11C. T. McTernan, J. A. Davies, J. R. Nitschke, Chem. Rev. 2022, 122, 10393–10437.
- 12D. Zhang, T. K. Ronson, J. R. Nitschke, Acc. Chem. Res. 2018, 51, 2423–2436.
- 13D. Ajami, L. Liu, J. Rebek Jr, Chem. Soc. Rev. 2015, 44, 490–499.
- 14D. S. Kim, J. L. Sessler, Chem. Soc. Rev. 2015, 44, 532–546.
- 15M. Yoshizawa, J. K. Klosterman, M. Fujita, Angew. Chem. Int. Ed. 2009, 48, 3418–3438.
- 16Y. He, J. Zhou, Y. Li, Y.-D. Yang, J. L. Sessler, X. Chi, J. Am. Chem. Soc. 2024, 146, 6225–6230.
- 17H. Wang, L. O. Jones, I. Hwang, M. J. Allen, D. Tao, V. M. Lynch, B. D. Freeman, N. M. Khashab, G. C. Schatz, Z. A. Page, J. L. Sessler, J. Am. Chem. Soc. 2021, 143, 20403–20410.
- 18W. Li, A. T. Bockus, B. Vinciguerra, L. Isaacs, A. R. Urbach, Chem. Commun. 2016, 52, 8537–8540.
- 19A. Fernando, T. L. Mako, A. M. Levenson, P. T. Cesana, A. M. Mendieta, J. M. Racicot, B. DeBoef, M. Levine, Supramol. Chem. 2019, 31, 545–557.
- 20K. Jie, Y. Zhou, H. P. Ryan, S. Dai, J. R. Nitschke, Adv. Mater. 2021, 33, 2005745.
- 21W. Liu, C. F. A. Gómez-Durán, B. D. Smith, J. Am. Chem. Soc. 2017, 139, 6390–6395.
- 22D. Zhang, M. A. Soto, L. Lewis, W. Y. Hamad, M. J. MacLachlan, Angew. Chem. Int. Ed. 2020, 59, 4705–4710.
- 23J. Tian, J. Ji, Y. Zhu, Y. He, H. Li, Y. Li, D. Luo, J. Xing, L. Qie, J. L. Sessler, X. Chi, Adv. Mater. 2024, 36, 2308507.
- 24I. Jahović, Y.-Q. Zou, S. Adorinni, J. R. Nitschke, S. Marchesan, Matter 2021, 4, 2123–2140.
- 25J. Uchida, M. Yoshio, S. Sato, H. Yokoyama, M. Fujita, T. Kato, Angew. Chem. Int. Ed. 2017, 56, 14085–14089.
- 26X. Zhang, D. Zhang, C. Wei, D. Wang, R. Lavendomme, S. Qi, Y. Zhu, J. Zhang, Y. Zhang, J. Wang, L. Xu, E.-Q. Gao, W. Yu, H.-B. Yang, M. He, Nat. Commun. 2024, 15, 3766.
- 27Y. Zhu, W. Zheng, W. Wang, H.-B. Yang, Chem. Soc. Rev. 2021, 50, 7395–7417.
- 28D. Zhang, T. K. Ronson, Y.-Q. Zou, J. R. Nitschke, Nat. Rev. Chem. 2021, 5, 168–182.
- 29M. Li, H. Zhu, S. Adorinni, W. Xue, A. Heard, A. M. Garcia, S. Kralj, J. R. Nitschke, S. Marchesan, Angew. Chem. Int. Ed. 2024, 63, e202406909.
- 30S. H. A. M. Leenders, R. Gramage-Doria, B. de Bruin, J. N. H. Reek, Chem. Soc. Rev. 2015, 44, 433–448.
- 31X. Yan, H. Wang, C. E. Hauke, T. R. Cook, M. Wang, M. L. Saha, Z. Zhou, M. Zhang, X. Li, F. Huang, P. J. Stang, J. Am. Chem. Soc. 2015, 137, 15276–15286.
- 32H. Vardhan, M. Yusubov, F. Verpoort, Coord. Chem. Rev. 2016, 306, 171–194.
- 33S. K. Samanta, L. Isaacs, Coord. Chem. Rev. 2020, 410, 213181.
- 34J. Liu, Z. Wang, P. Cheng, M. J. Zaworotko, Y. Chen, Z. Zhang, Nat. Rev. Chem. 2022, 6, 339–356.
- 35A. J. McConnell, Chem. Soc. Rev. 2022, 51, 2957–2971.
- 36J. A. Foster, R. M. Parker, A. M. Belenguer, N. Kishi, S. Sutton, C. Abell, J. R. Nitschke, J. Am. Chem. Soc. 2015, 137, 9722–9729.
- 37C. Lu, M. Zhang, D. Tang, X. Yan, Z. Zhang, Z. Zhou, B. Song, H. Wang, X. Li, S. Yin, H. Sepehrpour, P. J. Stang, J. Am. Chem. Soc. 2018, 140, 7674–7680.
- 38Q.-Q. Yan, L.-P. Zhou, H.-Y. Zhou, Z. Wang, L.-X. Cai, X.-Q. Guo, X.-Q. Sun, Q.-F. Sun, Dalton Trans. 2019, 48, 7080–7084.
- 39S. C. Wei, M. Pan, Y. Z. Fan, H. Liu, J. Zhang, C. Y. Su, Chem. - Eur. J. 2015, 21, 7418–7427.
- 40Z.-E. Zhang, Y.-F. Zhang, Y.-Z. Zhang, H.-L. Li, L.-Y. Sun, L.-J. Wang, Y.-F. Han, J. Am. Chem. Soc. 2023, 145, 7446–7453.
- 41A. V. Zhukhovitskiy, M. Zhong, E. G. Keeler, V. K. Michaelis, J. E. P. Sun, M. J. A. Hore, D. J. Pochan, R. G. Griffin, A. P. Willard, J. A. Johnson, Nat. Chem. 2016, 8, 33–41.
- 42Y. Gu, E. A. Alt, H. Wang, X. Li, A. P. Willard, J. A. Johnson, Nature 2018, 560, 65–69.
- 43S. Fajal, W. Mandal, S. Mollick, Y. D. More, A. Torris, S. Saurabh, M. M. Shirolkar, S. K. Ghosh, Angew. Chem. Int. Ed. 2022, 61, e202203385.
- 44J. Zhao, Y. Zhang, Z. Wang, D. Yang, Chem. Eur. J. 2025, 31, e202404363.
- 45M. Kieffer, A. M. Garcia, C. J. E. Haynes, S. Kralj, D. Iglesias, J. R. Nitschke, S. Marchesan, Angew. Chem. Int. Ed. 2019, 58, 7982–7986.
- 46L. Liang, W. Zhao, X.-J. Yang, B. Wu, Acc. Chem. Res. 2022, 55, 3218–3229.
- 47W. Zhang, J. Zhao, D. Yang, ChemPlusChem 2022, 87, e202200294.
- 48D. Yang, J. Zhao, X.-J. Yang, B. Wu, Org. Chem. Front. 2018, 5, 662–690.
- 49J. Zhao, D. Yang, X.-J. Yang, B. Wu, Coord. Chem. Rev. 2019, 378, 415–444.
- 50J. W. Steed, Chem. Soc. Rev. 2010, 39, 3686.
- 51L. Li, R. Sun, R. Zheng, Y. Huang, Mater. Des. 2021, 205, 109759.
- 52Y. Gao, J. Zhao, Z. Huang, T. K. Ronson, F. Zhao, Y. Wang, B. Li, C. Feng, Y. Yu, Y. Cheng, D. Yang, X.-J. Yang, B. Wu, Angew. Chem. Int. Ed. 2022, 61, e202201793.
- 53F. H. Beijer, R. P. Sijbesma, H. Kooijman, A. L. Spek, E. W. Meijer, J. Am. Chem. Soc. 1998, 120, 6761–6769.
- 54C. Zhao, J. Zhao, D. Yang, T. K. Ronson, L. Yu, H. Zhang, W. Zhang, F. Zhao, W. Sun, X.-J. Yang, B. Wu, CCS Chem. 2022, 4, 2043–2052.
- 55X. Fan, D. Zhang, S. Jiang, H. Wang, L.-T. Lin, B. Zheng, W.-H. Xu, Y. Zhao, B. P. Hay, Y.-T. Chan, X.-J. Yang, X. Li, B. Wu, Chem. Sci. 2019, 10, 6278–6284.
- 56X. Yan, S. Li, J. B. Pollock, T. R. Cook, J. Chen, Y. Zhang, X. Ji, Y. Yu, F. Huang, P. J. Stang, Proc. Natl. Acad. Sci. U.S.A. 2013, 110, 15585–15590.
- 57D. D. Allen, P. R. Lockman, Life Sci. 2003, 73, 1609–1615.
- 58A. K. Ghoshal, Crit. Rev. Biochem. Mol. Biol. 1995, 30, 263–273.
- 59S. H. Zeisel, K.-A. da Costa, Nutr. Rev. 2009, 67, 615–623.
- 60K. A. Shipkowski, J. M. Sanders, J. D. McDonald, C. E. Garner, M. Doyle-Eisele, C. J. Wegerski, S. Waidyanatha, Toxicol. Appl. Pharmacol. 2019, 378, 114592.