Dihydrophenazine Derived Pd6L12 Cage: Self-Assembly, Polyradical Cations, and Lithium Battery Cathode Application
Meng-Xiang Wu
State Key Laboratory of Petroleum Molecular & Process Engineering, Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai, 200062 China
Search for more papers by this authorYingli Li
College of Materials, Chemistry and Chemical Engineering, Hangzhou Normal University, 2318 Yuhangtang Road, Hangzhou, Zhejiang, 311121 China
Search for more papers by this authorJiefan Liu
Shanghai Key Laboratory of Magnetic Resonance, State Key Laboratory of Precision Spectroscopy, School of Physics and Electronic Science, East China Normal University, Shanghai, 200241 China
Search for more papers by this authorBin Huang
State Key Laboratory of Petroleum Molecular & Process Engineering, Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai, 200062 China
Search for more papers by this authorQiong-Yan Hong
State Key Laboratory of Petroleum Molecular & Process Engineering, Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai, 200062 China
Search for more papers by this authorWei-Ling Jiang
State Key Laboratory of Petroleum Molecular & Process Engineering, Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai, 200062 China
Search for more papers by this authorYu Zhao
College of Materials, Chemistry and Chemical Engineering, Hangzhou Normal University, 2318 Yuhangtang Road, Hangzhou, Zhejiang, 311121 China
Search for more papers by this authorCorresponding Author
Gaole Dai
College of Materials, Chemistry and Chemical Engineering, Hangzhou Normal University, 2318 Yuhangtang Road, Hangzhou, Zhejiang, 311121 China
E-mail: [email protected]; [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Bingwen Hu
Shanghai Key Laboratory of Magnetic Resonance, State Key Laboratory of Precision Spectroscopy, School of Physics and Electronic Science, East China Normal University, Shanghai, 200241 China
E-mail: [email protected]; [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Xueliang Shi
State Key Laboratory of Petroleum Molecular & Process Engineering, Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai, 200062 China
E-mail: [email protected]; [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Hai-Bo Yang
State Key Laboratory of Petroleum Molecular & Process Engineering, Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai, 200062 China
E-mail: [email protected]; [email protected]; [email protected]; [email protected]
Search for more papers by this authorMeng-Xiang Wu
State Key Laboratory of Petroleum Molecular & Process Engineering, Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai, 200062 China
Search for more papers by this authorYingli Li
College of Materials, Chemistry and Chemical Engineering, Hangzhou Normal University, 2318 Yuhangtang Road, Hangzhou, Zhejiang, 311121 China
Search for more papers by this authorJiefan Liu
Shanghai Key Laboratory of Magnetic Resonance, State Key Laboratory of Precision Spectroscopy, School of Physics and Electronic Science, East China Normal University, Shanghai, 200241 China
Search for more papers by this authorBin Huang
State Key Laboratory of Petroleum Molecular & Process Engineering, Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai, 200062 China
Search for more papers by this authorQiong-Yan Hong
State Key Laboratory of Petroleum Molecular & Process Engineering, Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai, 200062 China
Search for more papers by this authorWei-Ling Jiang
State Key Laboratory of Petroleum Molecular & Process Engineering, Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai, 200062 China
Search for more papers by this authorYu Zhao
College of Materials, Chemistry and Chemical Engineering, Hangzhou Normal University, 2318 Yuhangtang Road, Hangzhou, Zhejiang, 311121 China
Search for more papers by this authorCorresponding Author
Gaole Dai
College of Materials, Chemistry and Chemical Engineering, Hangzhou Normal University, 2318 Yuhangtang Road, Hangzhou, Zhejiang, 311121 China
E-mail: [email protected]; [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Bingwen Hu
Shanghai Key Laboratory of Magnetic Resonance, State Key Laboratory of Precision Spectroscopy, School of Physics and Electronic Science, East China Normal University, Shanghai, 200241 China
E-mail: [email protected]; [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Xueliang Shi
State Key Laboratory of Petroleum Molecular & Process Engineering, Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai, 200062 China
E-mail: [email protected]; [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Hai-Bo Yang
State Key Laboratory of Petroleum Molecular & Process Engineering, Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai, 200062 China
E-mail: [email protected]; [email protected]; [email protected]; [email protected]
Search for more papers by this authorGraphical Abstract
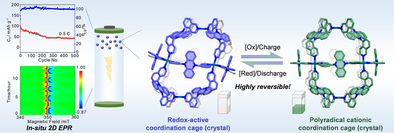
In this study, we synthesized a dihydrophenazine-based Pd6L12 coordination cage (Cage 1) with excellent redox activity. It can reversibly transform into its radical cation form (112•+) via electrochemical or chemical oxidation/reduction. Both forms' structures were determined by X-ray diffraction. In situ spectroelectrochemical techniques verified their redox reversibility and electrochromic behavior. Cage 1 was also used as a lithium battery cathode, with its redox behavior studied by in situ 2D EPR.
Abstract
In this study, we present the self-assembly of a dihydrophenazine-based Pd6L12-type coordination cage 1 showing excellent redox activity and demonstrate the use as the cathode for lithium batteries. The structure of cage 1 was confirmed by single-crystal X-ray diffraction analysis. The excellent reversible redox performance of 1 and its electrochromic properties induced by radical species were systematically characterized using in situ UV–vis–NIR and EPR spectroelectrochemistry. Notably, a highly stable radical cationic species 112•+, containing 12 radical cations, was successfully obtained through the chemical oxidation of 1, and its single-crystal structure was resolved. The excellent redox properties of 1 enable its application as a cathode material for lithium batteries. The 1|Li cell exhibited good cycling stability, nearly 100% coulombic efficiency, and an initial discharge capacity of 84 mAh g⁻¹ within a voltage range of 2.5–4.0 V. Furthermore, in situ 2D EPR experiments on lithium batteries visually revealed the excellent cycling stability of the 1-based cathode material and its reversible two-step electron transfer process. This study provides important insights into the design, synthesis, and properties of functionalized redox-active coordination cages, offering a reference for their application in energy storage and functional materials research.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
Data involved in this work are included in this article and the corresponding supplementary materials. Deposition numbers CCDC 2404229 (for L), 2404230 (for 1), 2404232 (for 112•+), 2433324 (for 1(PF6−)) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre Access Structures service.
Supporting Information
| Filename | Description |
|---|---|
| anie202503151-sup-0001-SuppMat.docx8.1 MB | Supporting Information |
| anie202503151-sup-0002-SuppMat.zip5 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Y. Liu, C. Hu, A. Comotti, M. D. Ward, Science 2011, 333, 436–440.
- 2N. Giri, M. G. Del Pópolo, G. Melaugh, R. L. Greenaway, K. Rätzke, T. Koschine, L. Pison, M. F. C. Gomes, A. I. Cooper, S. L. James, Nature 2015, 527, 216–220.
- 3D. M. Kaphan, M. D. Levin, R. G. Bergman, K. N. Raymond, F. D. Toste, Science 2015, 350, 1235–1238.
- 4D. Fujita, Y. Ueda, S. Sato, N. Mizuno, T. Kumasaka, M. Fujita, Nature 2016, 540, 563–566.
- 5D. Preston, J. J. Sutton, K. C. Gordon, J. D. Crowley, Angew. Chem. Int. Ed. 2018, 57, 8659–8663.
- 6M. Yamashina, Y. Tanaka, R. Lavendomme, T. K. Ronson, M. Pittelkow, J. R. Nitschke, Nature 2019, 574, 511–515.
- 7B. Li, W. Zhang, S. Lu, B. Zheng, D. Zhang, A. Li, X. Li, X.-J. Yang, B. Wu, J. Am. Chem. Soc. 2020, 142, 21160–21168.
- 8K. Wu, E. Benchimol, A. Baksi, G. H. Clever, Nat. Chem. 2024, 16, 584–591.
- 9A. Galan, P. Ballester, Chem. Soc. Rev. 2016, 45, 1720–1737.
- 10Y. Fang, J. A. Powell, E. Li, Q. Wang, Z. Perry, A. Kirchon, X. Yang, Z. Xiao, C. Zhu, L. Zhang, F. Huang, H.-C. Zhou, Chem. Soc. Rev. 2019, 48, 4707–4730.
- 11W.-X. Gao, H.-N. Zhang, G.-X. Jin, Coord. Chem. Rev. 2019, 386, 69–84.
- 12M. Morimoto, S. M. Bierschenk, K. T. Xia, R. G. Bergman, K. N. Raymond, F. D. Toste, Nat. Catal. 2020, 3, 969–984.
- 13S. P. Zheng, L. B. Huang, Z. Sun, M. Barboiu, Angew. Chem. Int. Ed. 2021, 60, 566–597.
- 14R. Ham, C. J. Nielsen, S. Pullen, J. N. H. Reek, Chem. Rev. 2023, 123, 5225–5261.
- 15D. Xu, Y. Li, S. Yin, F. Huang, Chem. Soc. Rev. 2024, 53, 3167–3204.
- 16R. A. Borse, Y.-X. Tan, D. Yuan, Y. Wang, Energy Environ. Sci. 2024, 17, 1307–1329.
- 17R. Banerjee, D. Chakraborty, P. S. Mukherjee, J. Am. Chem. Soc. 2023, 145, 7692–7711.
- 18S. Saha, I. Regeni, G. H. Clever, Coord. Chem. Rev. 2018, 374, 1–14.
- 19E. G. Percástegui, T. K. Ronson, J. R. Nitschke, Chem. Rev. 2020, 120, 13480–13544.
- 20Y.-L. Lu, Y.-P. Wang, K. Wu, M. Pan, C.-Y. Su, Acc. Chem. Res. 2024, 57, 3277–3291.
- 21H.-T. Feng, Y.-X. Yuan, J.-B. Xiong, Y.-S. Zheng, B. Z. Tang, Chem. Soc. Rev. 2018, 47, 7452–7476.
- 22J. Shi, W. Xu, H. Yu, X. Wang, F. Jin, Q. Zhang, H. Zhang, Q. Peng, A. Abdurahman, M. Wang, J. Am. Chem. Soc. 2023, 145, 24081–24088.
- 23H. Lee, J. Tessarolo, D. Langbehn, A. Baksi, R. Herges, G. H. Clever, J. Am. Chem. Soc. 2022, 144, 3099–3105.
- 24E. Benchimol, J. Tessarolo, G. H. Clever, Nat. Chem. 2024, 16, 13–21.
- 25X. Gu, T. Y. Gopalakrishna, H. Phan, Y. Ni, T. S. Herng, J. Ding, J. Wu, Angew. Chem. Int. Ed. 2017, 56, 15383–15387.
- 26T. Jiao, H. Qu, L. Tong, X. Cao, H. Li, Angew. Chem. Int. Ed. 2021, 60, 9852–9858.
- 27D. Zhang, T. K. Ronson, Y. Q. Zou, J. R. Nitschke, Nat. Rev. Chem. 2021, 5, 168–182.
- 28P. F. Cui, X. R. Liu, Y. J. Lin, Z. H. Li, G. X. Jin, J. Am. Chem. Soc. 2022, 144, 6558–6565.
- 29L. A. Pérez-Márquez, M. D. Perretti, R. Garcia-Rodriguez, F. Lahoz, R. Carrillo, Angew. Chem. Int. Ed. 2022, 61, e202205403.
- 30J. Wang, C. He, P. Wu, J. Wang, C. Duan, J. Am. Chem. Soc. 2011, 133, 12402–12405.
- 31H. Zhu, Q. Li, B. Shi, F. Ge, Y. Liu, Z. Mao, H. Zhu, S. Wang, G. Yu, F. Huang, P. J. Stang, Angew. Chem. Int. Ed. 2020, 59, 20208–20214.
- 32W.-T. Dou, C.-Y. Yang, L.-R. Hu, B. Song, T. Jin, P.-P. Jia, X. Ji, F. Zheng, H.-B. Yang, L. Xu, ACS Mater. Lett. 2023, 5, 1061–1082.
- 33T. R. Schulte, J. J. Holstein, G. H. Clever, Angew. Chem. Int. Ed. 2019, 58, 5562–5566.
- 34X. Tang, H. Jiang, Y. Si, N. Rampal, W. Gong, C. Cheng, X. Kang, D. Fairen-Jimenez, Y. Cui, Y. Liu, Chem 2021, 7, 2771–2786.
- 35X. Dong, H. Qu, A. C.-H. Sue, X.-C. Wang, X.-Y. Cao, Acc. Chem. Res. 2024, 57, 1111–1122.
- 36T. Li, Y. Pan, L. Ding, Y. Kang, X. Q. Hao, Y. Guo, L. Shi, Chem. Synth. 2024, 4, 35.
- 37G.-F. Huo, X. Shi, Q. Tu, Y.-X. Hu, G.-Y. Wu, G.-Q. Yin, X. Li, L. Xu, H.-M. Ding, H.-B. Yang, J. Am. Chem. Soc. 2019, 141, 16014–16023.
- 38W.-L. Jiang, Z. Peng, B. Huang, X.-L. Zhao, D. Sun, X. Shi, H.-B. Yang, J. Am. Chem. Soc. 2021, 143, 433–441.
- 39V. Croué, S. Goeb, G. Szalóki, M. Allain, M. Sallé, Angew. Chem. Int. Ed. 2016, 55, 1746–1750.
- 40G. Szalóki, V. Croué, V. Carré, F. Aubriet, O. Aléveque, E. Levillain, M. Allain, J. Aragó, E. Orti, S. Goeb, M. Sallé, Angew. Chem. Int. Ed. 2017, 56, 16272–16276.
- 41A. J. Plajer, F. J. Rizzuto, L. K. S. von Krbek, Y. Gisbert, V. Martínez-Agramunt, J. R. Nitschke, Chem. Sci. 2020, 11, 10399–10404.
- 42Z. Lu, T. K. Ronson, J. R. Nitschke, Chem. Sci. 2020, 11, 1097–1101.
- 43K. Hamashima, J. Yuasa, Angew. Chem. Int. Ed. 2022, 61, e202113914.
- 44J. Zheng, Y. Yang, T. K. Ronson, D. M. Wood, J. R. Nitschke, Adv. Mater. 2023, 35, 2302580.
- 45K. Mahata, P. D. Frischmann, F. Würthner, J. Am. Chem. Soc. 2013, 135, 15656–15661.
- 46R. D. Mukhopadhyay, Y. Kim, J. Koo, K. Kim, Acc. Chem. Res. 2018, 51, 2730–2738.
- 47Z. Lu, R. Lavendomme, O. Burghaus, J. R. Nitschke, Angew. Chem. Int. Ed. 2019, 58, 9073–9077.
- 48S. Bhattacharyya, S. R. Ali, M. Venkateswarulu, P. Howlader, E. Zangrando, M. De, P. S. Mukherjee, J. Am. Chem. Soc. 2020, 142, 18981–18989.
- 49C. Liu, K. Liu, C. Wang, H. Liu, H. Wang, H. Su, X. Li, B. Chen, J. Jiang, Nat. Commun. 2020, 11, 1047.
- 50H. Lin, Z. Xiao, K. N. Le, T.-h. Yan, P. Cai, Y. Yang, G. S. Day, H. F. Drake, H. Xie, R. Bose, C. A. Ryan, C. H. Hendon, H.-C. Zhou, Angew. Chem. Int. Ed. 2022, 61, e202214055.
- 51P. K. Maitra, S. Bhattacharyya, N. Hickey, P. S. Mukherjee, J. Am. Chem. Soc. 2024, 146, 15301–15308.
- 52S. K. Nalluri, Z. Liu, Y. Wu, K. R. Hermann, A. Samanta, D. J. Kim, M. D. Krzyaniak, M. R. Wasielewski, J. F. Stoddart, J. Am. Chem. Soc. 2016, 138, 5968–5977.
- 53D. J. Kim, K. R. Hermann, A. Prokofjevs, M. T. Otley, C. Pezzato, M. Owczarek, J. F. Stoddart, J. Am. Chem. Soc. 2017, 139, 6635–6643.
- 54D. J. Kim, D. J. Yoo, M. T. Otley, A. Prokofjevs, C. Pezzato, M. Owczarek, S. J. Lee, J. W. Choi, J. F. Stoddart, Nat. Energy 2019, 4, 51–59.
- 55S. Bera, N. Goujon, M. Melle-Franco, D. Mecerreyes, A. Mateo-Alonso, Chem. Sci. 2024, 15, 14872–14879.
- 56L. Mao, M. Zhou, T. Wu, D. Ma, G. Dai, X. Shi, Org. Lett. 2024, 26, 7244–7248.
- 57A. J. Gosselin, C. A. Rowland, E. D. Bloch, Chem. Rev. 2020, 120, 8987–9014.
- 58Y. Sun, C. Chen, J. Liu, P. J. Stang, Chem. Soc. Rev. 2020, 49, 3889–3919.
- 59D. Tripathy, N. B. Debata, K. C. Naik, H. S. Sahoo, Coord. Chem. Rev. 2022, 456, 214396.
- 60J. Liu, Z. Wang, P. Cheng, M. J. Zaworotko, Y. Chen, Z. Zhang, Nat. Rev. Chem. 2022, 6, 339–356.
- 61Y. Domoto, M. Fujita, Coord. Chem. Rev. 2022, 466, 214605.
- 62X. Z. Li, C. B. Tian, Q. F. Sun, Chem. Rev. 2022, 122, 6374–6458.
- 63J. Dong, Y. Liu, Y. Cui, Acc. Chem. Res. 2021, 54, 194–206.
- 64X.-W. Zhu, D. Luo, X.-P. Zhou, D. Li, Coord. Chem. Rev. 2022, 455, 214354.
- 65Q.-H. Ling, J.-L. Zhu, Y. Qin, L. Xu, Mater. Chem. Front. 2020, 4, 3176–3189.
- 66S. Bivaud, J.-Y. Balandier, M. Chas, M. Allain, S. Goeb, M. Sallé, J. Am. Chem. Soc. 2012, 134, 11968–11970.
- 67A. Jana, S. Bähring, M. Ishida, S. Goeb, D. Canevet, M. Sallé, J. O. Jeppesen, J. L. Sessler, Chem. Soc. Rev. 2018, 47, 5614–5645.
- 68M. Frank, J. Hey, I. Balcioglu, Y.-S. Chen, D. Stalke, T. Suenobu, S. Fukuzumi, H. Frauendorf, G. H. Clever, Angew. Chem. Int. Ed. 2013, 52, 10102–10106.
- 69E. G. Percástegui, V. Jancik, Coord. Chem. Rev. 2020, 407, 213165.
- 70K. G. Dutton, D. A. Rothschild, D. B. Pastore, T. J. Emge, M. C. Lipke, Inorg. Chem. 2020, 59, 12616–12624.
- 71B. Roy, E. Zangrando, P. S. Mukherjee, Chem. Commun. 2016, 52, 4489–4492.
- 72L. Zhao, J. Wei, J. Lu, C. He, C. Duan, Angew. Chem. Int. Ed. 2017, 56, 8692–8696.
- 73B. Huang, L. Mao, X. Shi, H.-B. Yang, Chem. Sci. 2021, 12, 13648–13663.
- 74Z. Zhang, X. Jin, X. Sun, J. Su, D.-H. Qu, Coord. Chem. Rev. 2022, 472, 214768.
- 75J. Dosso, M. Prato, Chem. - Eur. J. 2023, 29, e202203637.
- 76J. C. Theriot, C.-H. Lim, H. Yang, M. D. Ryan, C. B. Musgrave, G. M. Miyake, Science 2016, 352, 1082–1086.
- 77A. Bhattacherjee, M. Sneha, L. L. Borrell, G. Amoruso, T. A. A. Oliver, J. Tyler, I. P. Clark, A. J. Orr-Ewing, J. Am. Chem. Soc. 2021, 143, 3613–3627.
- 78K. Wang, X. Kang, C. Yuan, X. Han, Y. Liu, Y. Cui, Angew. Chem. Int. Ed. 2021, 60, 19466–19476.
- 79Y. Masuda, M. Kuratsu, S. Suzuki, M. Kozaki, D. Shiomi, K. Sato, T. Takui, Y. Hosokoshi, X.-Z. Lan, Y. Miyazaki, A. Inaba, K. Okada, J. Am. Chem. Soc. 2009, 131, 4670–4673.
- 80G. Dai, Y. He, Z. Niu, P. He, C. Zhang, Y. Zhao, X. Zhang, H. Zhou, Angew. Chem. Int. Ed. 2019, 58, 9902–9906.
- 81L. Li, Y. Su, Y. Ji, P. Wang, J. Am. Chem. Soc. 2023, 145, 5778–5785.
- 82Z. Zhang, Y.-S. Wu, K.-C. Tang, C.-L. Chen, J.-W. Ho, J. Su, H. Tian, P.-T. Chou, J. Am. Chem. Soc. 2015, 137, 8509–8520.
- 83Z. Huang, T. Jiang, J. Wang, X. Ma, H. Tian, Angew. Chem. Int. Ed. 2021, 60, 2855–2860.
- 84X. Jin, S. Li, L. Guo, J. Hua, D.-H. Qu, J. Su, Z. Zhang, H. Tian, J. Am. Chem. Soc. 2022, 144, 4883–4896.
- 85K. Yazaki, S. Noda, Y. Tanaka, Y. Sei, M. Akita, M. Yoshizawa, Angew. Chem. Int. Ed. 2016, 55, 15031–15034.
- 86Z. Zhou, D.-G. Chen, M. L. Saha, H. Wang, X. Li, P.-T. Chou, P. J. Stang, J. Am. Chem. Soc. 2019, 141, 5535–5543.
- 87W. Chen, C. Guo, Q. He, X. Chi, V. M. Lynch, Z. Zhang, J. Su, H. Tian, J. L. Sessler, J. Am. Chem. Soc. 2019, 141, 14798–14806.
- 88S. Yang, C.-X. Zhao, S. Crespi, X. Li, Q. Zhang, Z.-Y. Zhang, J. Mei, H. Tian, D.-H. Qu, Chem 2021, 7, 1544–1556.
- 89S. Qiu, Y. Zhao, L. Zhang, Y. Ni, Y. Wu, H. Cong, D.-H. Qu, W. Jiang, J. Wu, H. Tian, Z. Wang, CCS Chem 2023, 5, 1763–1772.
- 90S. An, K. Gong, C. Yang, J. Su, Z. Zhang, Chem. Eur. J. 2024, 30, e202400305.
- 91W.-L. Jiang, B. Huang, M.-X. Wu, Y.-K. Zhu, X.-L. Zhao, X. Shi, H.-B. Yang, Chem. Asian J. 2021, 16, 3985–3992.
- 92W.-L. Jiang, B. Huang, X.-L. Zhao, X. Shi, H.-B. Yang, Chem 2023, 9, 2655–2668.
- 93M.-X. Wu, Q.-Y. Hong, M. Li, W.-L. Jiang, B. Huang, S. Lu, H. Wang, H.-B. Yang, X.-L. Zhao, X. Shi, Chem. Commun. 2024, 60, 1184–1187.
- 94Q.-Y. Hong, B. Huang, M.-X. Wu, L. Xu, X.-L. Zhao, X. Shi, H.-B. Yang, Chin. J. Chem. 2024, 42, 1895–1900.
- 95B. Huang, M. Zhou, Q.-Y. Hong, M.-X. Wu, X.-L. Zhao, L. Xu, E.-Q. Gao, H.-B. Yang, X. Shi, Angew. Chem. Int. Ed. 2024, 63, e202407279.
- 96C. Gütz, R. Hovorka, C. Klein, Q. Q. Jiang, C. Bannwarth, M. Engeser, C. Schmuck, W. Assenmacher, W. Mader, F. Topić, K. Rissanen, S. Grimme, A. Lützen, Angew. Chem. Int. Ed. 2014, 53, 1693–1698.
- 97M. Xu, B. Sun, D. A. Poole, 3rd, E. O. Bobylev, X. Jing, J. Wu, C. He, C. Duan, J. N. H. Reek, Chem. Sci. 2023, 14, 11699–11707.
- 98J. D. Montmollin, A. B. Solea, D. W. Chen, F. Fadaei-Tirani, K. Severin, Inorg. Chem. 2024, 63, 4583–4588.
- 99B. Huang, H. Kang, X. L. Zhao, H. B. Yang, X. Shi, Cryst. Growth Des. 2022, 22, 3587–3593.
- 100J. Dosso, B. Bartolomei, N. Demitri, F. P. Cossío, M. Prato, J. Am. Chem. Soc. 2022, 144, 7295–7301.
- 101B. L. Bales, M. Meyer, S. Smith, M. Peric, J. Phys. Chem. A 2009, 113, 4930–4940.
- 102J. D. Nicholas, V. Chechik, J. Phys. Chem. B 2020, 124, 5646–5653.
- 103S. S. Eaton, K. M. More, B. M. Sawant, G. R. Eaton, J. Am. Chem. Soc. 1983, 105, 6560–6567.
- 104J. Telser, eMagRes 2017, 6, 207–233.
- 105Y. Bai, Z. Wang, N. Qin, D. Ma, W. Fu, Z. Lu, X. Pan, Angew. Chem. Int. Ed. 2023, 62, e202303162.
- 106M. Tang, A. Dalzini, X. Li, X. Feng, P.-H. Chien, L. Song, Y.-Y. Hu, J. Phys. Chem. Lett. 2017, 8, 4009–4016.
- 107F. Geng, Q. Yang, C. Li, B. Hu, C. Zhao, M. Shen, B. Hu, J. Phys. Chem. Lett. 2021, 12, 781–786.
- 108S. Künstner, J. E. McPeak, A. Chu, M. Kern, K.-P. Dinse, B. Naydenov, P. Fischer, J. Anders, K. Lips, Phys. Chem. Chem. Phys. 2024, 26, 17785–17795.
- 109R. Alcántara, P. Lavela, G. F. Ortiz, J. L. Tirado, R. Stoyanova, E. Zhecheva, C. Merino, Carbon 2004, 42, 2153–2161.
- 110B.-W. Hu, C. Li, F.-S. Geng, M. Shen, J. Electrochim. 2022, 28, 210842.