Plastic from CO2, Water, and Electricity: Tandem Electrochemical CO2 Reduction and Thermochemical Ethylene-CO Copolymerization
Maxim Zhelyabovskiy
Department of Chemistry and Chemical Engineering, California Institute of Technology, Pasadena, CA, 91125 USA
Search for more papers by this authorHyuk-Joon Jung
Department of Chemistry and Biochemistry, University of California Los Angeles, Los Angeles, CA, 90095 USA
Search for more papers by this authorPaula L. Diaconescu
Department of Chemistry and Biochemistry, University of California Los Angeles, Los Angeles, CA, 90095 USA
Search for more papers by this authorCorresponding Author
Jonas C. Peters
Department of Chemistry and Chemical Engineering, California Institute of Technology, Pasadena, CA, 91125 USA
E-mail: [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Theodor Agapie
Department of Chemistry and Chemical Engineering, California Institute of Technology, Pasadena, CA, 91125 USA
E-mail: [email protected]; [email protected]
Search for more papers by this authorMaxim Zhelyabovskiy
Department of Chemistry and Chemical Engineering, California Institute of Technology, Pasadena, CA, 91125 USA
Search for more papers by this authorHyuk-Joon Jung
Department of Chemistry and Biochemistry, University of California Los Angeles, Los Angeles, CA, 90095 USA
Search for more papers by this authorPaula L. Diaconescu
Department of Chemistry and Biochemistry, University of California Los Angeles, Los Angeles, CA, 90095 USA
Search for more papers by this authorCorresponding Author
Jonas C. Peters
Department of Chemistry and Chemical Engineering, California Institute of Technology, Pasadena, CA, 91125 USA
E-mail: [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Theodor Agapie
Department of Chemistry and Chemical Engineering, California Institute of Technology, Pasadena, CA, 91125 USA
E-mail: [email protected]; [email protected]
Search for more papers by this authorGraphical Abstract
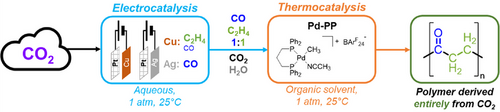
A new system of generating entirely CO2-generated abiotic polymers by integrating electrochemical CO2 reduction (eCO2R) and organometallic polymerization catalysis is presented, having an overall CO2-to-polymer conversion of 14% and current-to-polymer efficiency of 51%. A strategy for tuning the product composition obtained from eCO2R by combining different electrocatalysts is introduced. CO-ethylene copolymerization activity in the presence of potential poisons from eCO2R is retained.
Abstract
Converting CO2 into industrially useful products is an appealing strategy for utilization of an abundant chemical resource. Electrochemical CO2 reduction (eCO2R) offers a pathway to convert CO2 into CO and ethylene, using renewable electricity. These products can be efficiently copolymerized by organometallic catalysts to generate polyketones. However, the conditions for these reactions are very different, presenting the challenge of coupling microenvironments typically encountered for the transformation of CO2 into highly complex but desirable multicarbon products. Herein, we present a system to produce polyketone plastics entirely derived from CO2 and water, where both the CO and C2H4 intermediates are produced by eCO2R. In this system, a combination of Cu and Ag gas diffusion electrodes is used to generate a gas mixture with nearly equal concentrations of CO and C2H4, and a recirculatory CO2 reduction loop is used to reach concentrations of above 11% each, leading to a current-to-polymer efficiency of up to 51% and CO2 utilization of 14%.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
Supporting Information
| Filename | Description |
|---|---|
| anie202503003-sup-0001-SuppMat.pdf21.2 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Y. Zhu, C. Romain, C. K. Williams, Nature 2016, 540, 354–362.
- 2X. Zhang, M. Fevre, G. O. Jones, R. M. Waymouth, Chem. Rev. 2018, 118, 839–885.
- 3P. De Luna, C. Hahn, D. Higgins, S. A. Jaffer, T. F. Jaramillo, E. H. Sargent, Science 2019, 26, 364.
- 4J. Artz, T. E. Müller, K. Thenert, J. Kleinekorte, R. Meys, A. Sternberg, A. Bardow, W. Leitner, Chem. Rev. 2018, 118, 434–504.
- 5F. Keller, R. P. Lee, B. Meyer, J. Clean Prod. 2020, 250, 119484.
- 6V. P. Haribal, Y. Chen, L. Neal, F. Li, Engineering 2018, 4, 714–721.
- 7R. Geyer, J. R. Jambeck, K. L. Law, Sci. Adv. 2017, 3, e1700782.
- 8B. Grignard, S. Gennen, C. Jérôme, A. W. Kleij, C. Detrembleur, Chem. Soc. Rev. 2019, 48, 4466–4514.
- 9C. Yokoyama, Y. Kawase, N. Shibasaki-Kitakawa, R. L. Smith, J. Appl. Polym. Sci. 2003, 89, 3167–3174.
- 10K. Soga, M. Sato, S. Hosoda, S. Ikeda, J. Polym. Sci. Polym. Lett. Ed. 1975, 13, 543–548.
- 11M. Tamura, K. Ito, M. Honda, Y. Nakagawa, H. Sugimoto, K. Tomishige, Sci. Rep. 2016, 6, 24038.
- 12G. P. Wu, S. H. Wei, W. M. Ren, X. B. Lu, T. Q. Xu, D. J. Darensbourg, J. Am. Chem. Soc. 2011, 133, 15191–15199.
- 13B. Li, R. Zhang, X. B. Lu, Macromolecules 2007, 40, 2303–2307.
- 14S. D. Allen, D. R. Moore, E. B. Lobkovsky, G. W. Coates, J. Am. Chem. Soc. 2002, 124, 14284–14285.
- 15Y. Wang, D. J. Darensbourg, Coord. Chem. Rev. 2018, 372, 85–100.
- 16G. W. Coates, D. R. Moore, Angew. Chem. Int. Ed. 2004, 43, 6618–6639.
- 17S. Tang, K. Nozaki, Acc. Chem. Res. 2022, 55, 1524–1532.
- 18R. Nakano, S. Ito, K. Nozaki, Nat. Chem. 2014, 6, 325–331.
- 19S. Yue, T. Bai, S. Xu, T. Shen, J. Ling, X. Ni, ACS Macro Lett. 2021, 10, 1055–1060.
- 20S. Klaus, M. W. Lehenmeier, C. E. Anderson, B. Rieger, Coord. Chem. Rev. 2011, 255, 1460–1479.
- 21S. Elmas, M. A. Subhani, H. Vogt, W. Leitner, T. E. Müller, Green Chem. 2013, 15, 1356–1360.
- 22C. J. Price, B. J. E. Reich, S. A. Miller, Macromolecules 2006, 39, 2751–2756.
- 23M. Cokoja, C. Bruckmeier, B. Rieger, W. A. Herrmann, F. E. Kühn, Angew. Chem. Int. Ed. 2011, 50, 8510–8537.
- 24S. Chu, Y. Cui, N. Liu, Nat. Mater. 2017, 16, 16–22.
- 25M. Schreier, F. Héroguel, L. Steier, S. Ahmad, J. S. Luterbacher, M. T. Mayer, J. Luo, M. Grätzel, Nat. Energy 2017, 2, 17087.
- 26S. Nitopi, E. Bertheussen, S. B. Scott, X. Liu, A. K. Engstfeld, S. Horch, B. Seger, I. E. L. Stephens, K. Chan, C. Hahn, J. K. Nørskov, T. F. Jaramillo, I. Chorkendorff, Chem. Rev. 2019, 119, 7610–7672.
- 27M. G. Lee, X.-Y. Li, A. Ozden, J. Wicks, P. Ou, Y. Li, R. Dorakhan, J. Lee, H. K. Park, J. W. Yang, B. Chen, J. Abed, R. dos Reis, G. Lee, J. E. Huang, T. Peng, Y.-H. Chin, D. Sinton, E. H. Sargent, Nat. Catal. 2023, 6, 310–318.
- 28H. M. Dodge, B. S. Natinsky, B. J. Jolly, H. Zhang, Y. Mu, S. M. Chapp, T. V. Tran, P. L. Diaconescu, L. H. Do, D. Wang, C. Liu, A. J. M. Miller, ACS Catal. 2023, 13, 4053–4059.
- 29J. Zhang, X. Kang, Y. Yan, X. Ding, L. He, Y. Li, Angew. Chem. Int. Ed. 2024, 63, e202315777.
- 30A. N. Biswas, Z. Xie, R. Xia, S. Overa, F. Jiao, J. G. Chen, ACS Energy Lett. 2022, 7, 2904–2910.
- 31A. Vavasori, L. Ronchin, Polyketones: Synthesis and Applications, In Encyclopedia of Polymer Science and Technology, John Wiley & Sons, Hoboken, NJ 2017, pp. 1–41.
- 32E. Drent, W. P. Mul, in Encyclopedia of Polymer Science and Technology, Vol. 3, John Wiley & Sons, Hoboken, NJ 2002, pp. 678–693.
- 33T. Morita, R. Taniguchi, J. Kato, Polym. J. 2004, 36, 495–497.
- 34B. J. Lommerts, E. A. Klop, J. Aerts, J. Polym. Sci. B Polym. Phys. 1993, 31, 1319–1330.
- 35A. Asano, M. Nishioka, Y. Takahashi, A. Kato, S. Hikasa, H. Iwabuki, K. Nagata, H. Sato, T. Hasegawa, H. Sawabe, M. Arao, T. Suda, A. Isoda, M. Mukai, D. Ishikawa, T. Izumi, Macromolecules 2009, 42, 9506–9514.
- 36A. Kato, M. Nishioka, Y. Takahashi, T. Suda, H. Sawabe, A. Isoda, O. Drozdova, T. Hasegawa, T. Izumi, K. Nagata, S. Hikasa, H. Iwabuki, A. Asano, J. Appl. Polym. Sci. 2010, 116, 3056–3069.
- 37T. O. Morgen, M. Baur, I. Göttker-Schnetmann, S. Mecking, Nat. Commun. 2020, 11, 3693.
- 38M. Baur, F. Lin, T. O. Morgen, L. Odenwald, S. Mecking, Science. 2021, 374, 604–607.
- 39K. P. Kuhl, E. R. Cave, D. N. Abram, T. F. Jaramillo, Energy Environ. Sci. 2012, 5, 7050–7059.
- 40H. Noda, S. Ikeda, Y. Oda, K. Imai, M. Maeda, K. Ito, Bull. Chem. Soc. Jpn. 1990, 63, 2459–2462.
- 41C.-T. Dinh, T. Burdyny, M. G. Kibria, A. Seifitokaldani, C. M. Gabardo, F. P. García de Arquer, A. Kiani, J. P. Edwards, P. De Luna, O. S. Bushuyev, C. Zou, R. Quintero-Bermudez, Y. Pang, D. Sinton, E. H. Sargent, Science 2018, 360, 783–787.
- 42C. Reller, R. Krause, E. Volkova, B. Schmid, S. Neubauer, A. Rucki, M. Schuster, G. Schmid, Adv. Energy Mater. 2017, 7, 1602114.
- 43Y. Hori, K. Kikuchi, S. Suzuki, Chem. Lett. 1985, 14, 1695–1698.
- 44D. Wakerley, S. Lamaison, J. Wicks, A. Clemens, J. Feaster, D. Corral, S. A. Jaffer, A. Sarkar, M. Fontecave, E. B. Duoss, S. Baker, E. H. Sargent, T. F. Jaramillo, C. Hahn, Nat. Energy 2022, 7, 130–143.
- 45M. Sassenburg, R. de Rooij, N. T. Nesbitt, R. Kas, S. Chandrashekar, N. J. Firet, K. Yang, K. Liu, M. A. Blommaert, M. Kolen, D. Ripepi, W. A. Smith, T. Burdyny, ACS Appl. Energy Mater. 2022, 5, 5983–5994.
- 46X. Lu, C. Zhu, Z. Wu, J. Xuan, J. S. Francisco, H. Wang, J. Am. Chem. Soc. 2020, 142, 15438–15444.
- 47T. K. Todorova, M. W. Schreiber, M. Fontecave, ACS Catal. 2020, 10, 1754–1768.
- 48D. A. Henckel, M. J. Counihan, H. E. Holmes, X. Chen, U. O. Nwabara, S. Verma, J. Rodríguez-López, P. J. A. Kenis, A. A. Gewirth, ACS Catal. 2021, 11, 255–263.
- 49A. S. Abu-Surrah, B. Rieger, Top. Catal. 1999, 7, 165–177.
- 50K. A. Alferov, O. M. Chukanova, G. P. Belov, Russ. Chem. Bull. 2011, 60, 1858–1861.
- 51C. Bianchini, A. Meli, Coord. Chem. Rev. 2002, 225, 35–66.
- 52B. De Mot, J. Hereijgers, M. Duarte, T. Breugelmans, Chem. Eng. J. 2019, 378, 122224.
- 53K. Yang, R. Kas, W. A. Smith, T. Burdyny, ACS Energy Lett. 2021, 6, 33–40.
- 54B. Endrődi, G. Bencsik, F. Darvas, R. Jones, K. Rajeshwar, C. Janáky, Prog. Energy Combust. Sci. 2017, 62, 133–154.
- 55E. Drent, J. A. M. Van Broekhoven, M. J. Doyle, J. Organomet. Chem. 1991, 417, 235–251.
- 56E. Drent, P. H. M. Budzelaar, Chem. Rev. 1996, 96, 663–682.
- 57S. Ma, M. Sadakiyo, R. Luo, M. Heima, M. Yamauchi, P. J. A. Kenis, J. Power Sources 2016, 301, 219–228.
- 58L. M. Baumgartner, C. I. Koopman, A. Forner-Cuenca, D. A. Vermaas, ACS Appl. Energy Mater. 2022, 5, 15125–15135.
- 59Y. Xu, J. P. Edwards, S. Liu, R. K. Miao, J. E. Huang, C. M. Gabardo, C. P. O'Brien, J. Li, E. H. Sargent, D. Sinton, ACS Energy Lett. 2021, 6, 809–815.
- 60H. Bao, Y. Qiu, X. Peng, J. Wang, Y. Mi, S. Zhao, X. Liu, Y. Liu, R. Cao, L. Zhuo, J. Ren, J. Sun, J. Luo, X. Sun, Nat. Commun. 2021, 12, 238.
- 61S. Li, A. Guan, C. Yang, C. Peng, X. Lv, Y. Ji, Y. Quan, Q. Wang, L. Zhang, G. Zheng, ACS Mater. Lett. 2021, 3, 1729–1737.
- 62C. S. Shultz, J. Ledford, J. M. DeSimone, M. Brookhart, J. Am. Chem. Soc. 2000, 122, 6351–6356.
- 63D. S. Ripatti, T. R. Veltman, M. W. Kanan, Joule 2019, 3, 240–256.
- 64K. Xie, R. K. Miao, A. Ozden, S. Liu, Z. Chen, C.-T. Dinh, J. E. Huang, Q. Xu, C. M. Gabardo, G. Lee, J. P. Edwards, C. P. O'Brien, S. W. Boettcher, D. Sinton, E. H. Sargent, Nat. Commun. 2022, 13, 3609.
- 65J. A. Rabinowitz, M. W. Kanan, Nat. Commun. 2020, 11, 5231.
- 66P. W. N. M. van Leeuwen, in Catalytic Synthesis of Alkene-Carbon Monoxide Copolymers and Cooligomers, Springer, Boston, MA 2003, pp. 141–188.
10.1007/978-1-4419-9266-6_5 Google Scholar
- 67B. De Mot, J. Hereijgers, M. Duarte, T. Breugelmans, Chem. Eng. J. 2019, 378, 122224.
- 68P. Mardle, S. Cassegrain, F. Habibzadeh, Z. Shi, S. Holdcroft, J. Phys. Chem. C 2021, 46, 25446–25454.
- 69T. Zhang, Z. Li, X. Lyu, J. Raj, G. Zhang, H. Kim, X. Wang, S. Chae, L. Lemen, V. N. Shanov, J. Wu, J. Electrochem. Soc. 2022, 169, 104506.
- 70T. Fujita, K. Nakano, M. Yamashita, K. Nozaki, J. Am. Chem. Soc. 2006, 128, 1968–1975.
- 71G. P. C. M. Dekker, C. J. Elsevier, K. Vrieze, P. W. N. M. Van Leeuwen. Organometallics 1992, 11, 1598–1603.
- 72N. A. Yakelis, R. G. Bergman, Organometallics 2005, 24, 3579–3581.
- 73P. Lobaccaro, M. R. Singh, E. L. Clark, Y. Kwon, A. T. Bell, J. W. Ager, Phys. Chem. Chem. Phys. 2016, 18, 26777–26785.