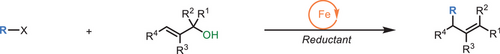Iron-Catalyzed Radical Allylic Substitution of Unprotected Allylic Alcohols
Gang Liu
Zhejiang Key Laboratory of Precise Synthesis of Functional Molecules of Zhejiang Province, Department of Chemistry, School of Science and Research Center for Industries of the Future, Westlake University, Hangzhou, Zhejiang Province, 310030 China
Search for more papers by this authorKe Gao
Zhejiang Key Laboratory of Precise Synthesis of Functional Molecules of Zhejiang Province, Department of Chemistry, School of Science and Research Center for Industries of the Future, Westlake University, Hangzhou, Zhejiang Province, 310030 China
Search for more papers by this authorTianbing Yao
Zhejiang Key Laboratory of Precise Synthesis of Functional Molecules of Zhejiang Province, Department of Chemistry, School of Science and Research Center for Industries of the Future, Westlake University, Hangzhou, Zhejiang Province, 310030 China
Search for more papers by this authorHui Hu
Zhejiang Key Laboratory of Precise Synthesis of Functional Molecules of Zhejiang Province, Department of Chemistry, School of Science and Research Center for Industries of the Future, Westlake University, Hangzhou, Zhejiang Province, 310030 China
Institute of Natural Sciences, Westlake Institute for Advanced Study, Hangzhou, Zhejiang Province, 310030 China
Search for more papers by this authorCorresponding Author
Zhaobin Wang
Zhejiang Key Laboratory of Precise Synthesis of Functional Molecules of Zhejiang Province, Department of Chemistry, School of Science and Research Center for Industries of the Future, Westlake University, Hangzhou, Zhejiang Province, 310030 China
Institute of Natural Sciences, Westlake Institute for Advanced Study, Hangzhou, Zhejiang Province, 310030 China
State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210093 China
E-mail: [email protected]
Search for more papers by this authorGang Liu
Zhejiang Key Laboratory of Precise Synthesis of Functional Molecules of Zhejiang Province, Department of Chemistry, School of Science and Research Center for Industries of the Future, Westlake University, Hangzhou, Zhejiang Province, 310030 China
Search for more papers by this authorKe Gao
Zhejiang Key Laboratory of Precise Synthesis of Functional Molecules of Zhejiang Province, Department of Chemistry, School of Science and Research Center for Industries of the Future, Westlake University, Hangzhou, Zhejiang Province, 310030 China
Search for more papers by this authorTianbing Yao
Zhejiang Key Laboratory of Precise Synthesis of Functional Molecules of Zhejiang Province, Department of Chemistry, School of Science and Research Center for Industries of the Future, Westlake University, Hangzhou, Zhejiang Province, 310030 China
Search for more papers by this authorHui Hu
Zhejiang Key Laboratory of Precise Synthesis of Functional Molecules of Zhejiang Province, Department of Chemistry, School of Science and Research Center for Industries of the Future, Westlake University, Hangzhou, Zhejiang Province, 310030 China
Institute of Natural Sciences, Westlake Institute for Advanced Study, Hangzhou, Zhejiang Province, 310030 China
Search for more papers by this authorCorresponding Author
Zhaobin Wang
Zhejiang Key Laboratory of Precise Synthesis of Functional Molecules of Zhejiang Province, Department of Chemistry, School of Science and Research Center for Industries of the Future, Westlake University, Hangzhou, Zhejiang Province, 310030 China
Institute of Natural Sciences, Westlake Institute for Advanced Study, Hangzhou, Zhejiang Province, 310030 China
State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210093 China
E-mail: [email protected]
Search for more papers by this authorGraphical Abstract
We report an iron-catalyzed radical allylic substitution method that directly functionalizes unprotected allylic alcohols, eliminating the need for prefunctionalization and toxic reagents. Utilizing iron's unique redox and oxophilic properties, this approach offers a step-economic and sustainable pathway to allylic functionalization. This method expands the synthetic toolbox and demonstrates the versatility of outer-sphere mechanisms in radical-based reactions, facilitating the efficient synthesis of diverse molecular architectures.
Abstract
Allylic substitution reactions are essential in organic synthesis, enabling the transformation of allylic reagents into diverse alkenes. Traditional methods, which typically operate through ionic pathways, often require substrate preactivation to address high C─O bond dissociation energies, leading to challenges in regioselectivity and limited substrate compatibility. Here, we introduce an iron-catalyzed radical pathway for allylic substitution that directly activates unprotected allylic alcohols, leveraging the redox and oxophilic properties of low-valent iron to promote selective C─O bond cleavage and allylic transposition. This radical approach achieves high regio- and stereoselectivity, providing access to a broad array of di-, tri-, and tetra-substituted alkenes with moderate to excellent yields and exceptional E/Z selectivity. Mechanistic studies confirm that the iron catalyst generates radical intermediates and mediates efficient dehydroxylation, enabling this direct transformation without protective groups or Lewis acid activators. The method's versatility is demonstrated through a broad substrate scope, including complex natural derivatives and functionalized alkyl halides, along with successful gram-scale synthesis and downstream derivatization. This iron-catalyzed radical pathway offers a sustainable and efficient alternative to conventional ionic methods, expanding the scope of allylic substitutions and advancing radical-based methodologies in synthetic chemistry.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
Supporting Information
| Filename | Description |
|---|---|
| anie202500781-supp-0001-SuppMat.docx12 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1K. Nogi, H. Yorimitsu, Chem. Rev. 2021, 121, 345–364.
- 2I. Volchkov, D. Lee, Chem. Soc. Rev. 2014, 43, 4381.
- 3D. A. Alonso, B. Maciá, I. M. Pastor, A. Baeza, ACS Org. Inorg. Au 2024, 4, 269–286.
- 4B. Sundararaju, M. Achard, C. Bruneau, Chem. Soc. Rev. 2012, 41, 4467.
- 5N. A. Butt, W. Zhang, Chem. Soc. Rev. 2015, 44, 7929–7967.
- 6N. Astrain-Redin, C. Sanmartin, A. K. Sharma, D. Plano, J. Med. Chem. 2023, 66, 3703–3731.
- 7A. B. Flynn, W. W. Ogilvie, Chem. Rev. 2007, 107, 4698–4745.
- 8T. Neveselý, M. Wienhold, J. J. Molloy, R. Gilmour, Chem. Rev. 2022, 122, 2650–2694.
- 9M.-Z. Lu, J. Goh, M. Maraswami, Z. Jia, J.-S. Tian, T.-P. Loh, Chem. Rev. 2022, 122, 17479–17646.
- 10H. Jiang, A. Studer, Chem. Soc. Rev. 2020, 49, 1790–1811.
- 11S. Vivek Kumar, A. Yen, M. Lautens, P. J. Guiry, Chem. Soc. Rev. 2021, 50, 3013–3093.
- 12B. M. Trost, D. L. van Vranken, Chem. Rev. 1996, 96, 395–422.
- 13B. M. Trost, M. L. Crawley, Chem. Rev. 2003, 103, 2921–2944.
- 14L. Süsse, B. M. Stoltz, Chem. Rev. 2021, 121, 4084–4099.
- 15Q. Cheng, H.-F. Tu, C. Zheng, J.-P. Qu, G. Helmchen, S.-L. You, Chem. Rev. 2019, 119, 1855–1969.
- 16J. D. Weaver, A. Recio, III, A. J. Grenning, J. A. Tunge, Chem. Rev. 2011, 111, 1846–1913.
- 17H.-M. Huang, P. Bellotti, F. Glorius, Chem. Soc. Rev. 2020, 49, 6186–6197.
- 18E. Emer, R. Sinisi, M. G. Capdevila, D. Petruzziello, F. de Vincentiis, P. G. Cozzi, Eur. J. Org. Chem. 2011, 2011, 647–666.
- 19G. Li, X. Huo, X. Jiang, W. Zhang, Chem. Soc. Rev. 2020, 49, 2060–2118.
- 20A. A. Zemtsov, V. V. Levin, A. D. Dilman, Coordin. Chem. Rev. 2022, 459, 214455.
- 21J. F. Hartwig, L. M. Stanley, Acc. Chem. Res. 2010, 43, 1461–1475.
- 22J. Qu, G. Helmchen, Acc. Chem. Res. 2017, 50, 2539–2555.
- 23J. Tsuji, H. Takahashi, M. Morikawa, Tetrahedron Lett. 1965, 6, 4387–4388.
- 24B. M. Trost, T. J. Fullerton, J. Am. Chem. Soc. 1973, 95, 292–294.
- 25O. Pàmies, J. Margalef, S. Cañellas, J. James, E. Judge, P. J. Guiry, C. Moberg, J.-E. Bäckvall, A. Pfaltz, M. A. Pericàs, M. Diéguez, Chem. Rev. 2021, 121, 4373–4505.
- 26M. D. Johnson, Acc. Chem. Res. 1983, 16, 343–349.
- 27J. C. Walton, Acc. Chem. Res. 1998, 31, 99–107.
- 28G. C. Fu, ACS Cent. Sci. 2017, 3, 692–700.
- 29F. Wang, P. Chen, G. Liu, Acc. Chem. Res. 2018, 51, 2036–2046.
- 30X.-Y. Dong, Z.-L. Li, Q.-S. Gu, X.-Y. Liu, J. Am. Chem. Soc. 2022, 144, 17319–17329.
- 31W.-C. C. Lee, X. P. Zhang, Angew. Chem. Int. Ed. 2024, 63, e202320243.
- 32M. Yan, J. C. Lo, J. T. Edwards, P. S. Baran, J. Am. Chem. Soc. 2016, 138, 12692–12714.
- 33J. Grignon, M. Pereyre, J. Organomet. Chem. 1973, 61, C33–C35.
- 34M. Kosugi, K. Kurino, K. Takayama, T. Migita, J. Organomet. Chem. 1973, 56, C11–C13.
- 35G. E. Keck, J. B. Yates, J. Am. Chem. Soc. 1982, 104, 5829–5831.
- 36J. Shen, Z. Xu, S. Yang, S. Li, J. Jiang, Y.-Q. Zhang, J. Am. Chem. Soc. 2023, 145, 21122–21131.
- 37Y. Guindon, B. Guérin, C. Chabot, W. Ogilvie, J. Am. Chem. Soc. 1996, 118, 12528–12535.
- 38B. Cardinal-David, B. Guérin, Y. Guindon, J. Org. Chem. 2005, 70, 776–784.
- 39C. C. Huval, D. A. Singleton, Tetrahedron Lett. 1993, 34, 3041–3042.
- 40T. Kippo, K. Hamaoka, I. Ryu, J. Am. Chem. Soc. 2013, 135, 632–635.
- 41L. Debien, B. Quiclet-Sire, S. Z. Zard, Acc. Chem. Res. 2015, 48, 1237–1253.
- 42B. Li, W. Zeng, L. Wang, Z. Geng, T.-P. Loh, P. Xie, Org. Lett. 2021, 23, 5235–5240.
- 43H.-H. Zhang, J.-J. Zhao, S. Yu, J. Am. Chem. Soc. 2018, 140, 16914–16919.
- 44G. Guo, X. Li, Tetrahedron 2023, 142, 133520.
- 45D.-R. Zhang, L.-P. Hu, F.-L. Liu, X.-H. Huang, X. Li, B. Liu, M.-Y. Teng, G.-L. Huang, Org. Chem. Front. 2023, 10, 4927–4934.
- 46F.-L. Haut, R. S. Mega, J. V. Estornell, R. Martin, Angew. Chem. Int. Ed. 2023, 62, e202304084.
- 47J. Ni, X. Xia, W.-F. Zheng, Z. Wang, J. Am. Chem. Soc. 2022, 144, 7889–7900.
- 48H. Hu, Z. Wang, J. Am. Chem. Soc. 2023, 145, 20775–20781.
- 49H. Shen, Z. Zhang, Z. Shi, K. Gao, Z. Wang, Chem 2024, 10, 998–1014.
- 50S. J. Blanksby, G. B. Ellison, Acc. Chem. Res. 2003, 36, 255–263.
- 51M. D. Morse, Acc. Chem. Res. 2019, 52, 119–126.
- 52N. G. Boekell, R. A. Flowers, Chem. Rev. 2022, 122, 13447–13477.
- 53A. F. Barrero, J. E. Oltra, J. M. Cuerva, A. Rosales, J. Org. Chem. 2002, 67, 2566–2571.
- 54D. A. Spiegel, K. B. Wiberg, L. N. Schacherer, M. R. Medeiros, J. L. Wood, J. Am. Chem. Soc. 2005, 127, 12513–12515.
- 55H. Lindner, W. M. Amberg, E. M. Carreira, J. Am. Chem. Soc. 2023, 145, 22347–22353.
- 56E. P. Beaumier, A. J. Pearce, X. Y. See, I. A. Tonks, Nat. Rev. Chem. 2019, 3, 15–34.
- 57R. M. Bullock, J. G. Chen, L. Gagliardi, P. J. Chirik, O. K. Farha, C. H. Hendon, C. W. Jones, J. A. Keith, J. Klosin, S. D. Minteer, R. H. Morris, A. T. Radosevich, T. B. Rauchfuss, N. A. Strotman, A. Vojvodic, T. R. Ward, J. Y. Yang, Y. Surendranath, Science 2020, 369, eabc3183.
- 58U. Jana, S. Maiti, S. Biswas, Tetrahedron Lett. 2007, 48, 7160–7163.
- 59U. Jana, S. Biswas, S. Maiti, Tetrahedron Lett. 2007, 48, 4065–4069.
- 60R. A. Watile, A. Bunrit, J. Margalef, S. Akkarasamiyo, R. Ayub, E. Lagerspets, S. Biswas, T. Repo, J. S. M. Samec, Nat. Commun. 2019, 10, 3826–3835.
- 61P. T. Marcyk, L. R. Jefferies, D. I. AbuSalim, M. Pink, M.-H. Baik, S. P. Cook, Angew. Chem. Int. Ed. 2019, 58, 1727–1731.
- 62C. Bolm, J. Legros, J. Le Paih, L. Zani, Chem. Rev. 2004, 104, 6217–6254.
- 63I. Bauer, H.-J. Knölker, Chem. Rev. 2015, 115, 3170–3387.
- 64A. Fürstner, ACS Cent. Sci. 2016, 2, 778–789.
- 65G. S. Ghotekar, M. Mujahid, M. Muthukrishnan, ACS Omega 2019, 4, 1322–1328.
- 66S. J. Park, J. Kim, J. Kim, Y. Kim, E. H. Lee, H. J. Kim, S. Kim, B. Kim, R. Kim, J. W. Choi, J.-H. Park, K. D. Park, Molecules 2022, 27, 2818.
- 67O. Buriez, M. Durandetti, J. Périchon, J. Electroanal. Chem. 2005, 578, 63–70.
- 68C. Zhang, L. Wang, H. Shi, Z. Lin, C. Wang, Org. Lett. 2022, 24, 3211–3216.
- 69B. Yuan, C. Zhang, H. Dong, C. Wang, Org. Lett. 2023, 25, 1883–1888.
- 70J. Shao, K. Richards, D. Rawlins, B. Han, C. A. Hansen, J. Porphyrins Phthalocyanines 2013, 17, 317–330.
- 71D. E. Essayan, M. J. Schubach, J. M. Smoot, T. Puri, S. V. Pronin, J. Am. Chem. Soc. 2024, 146, 18224–18229.