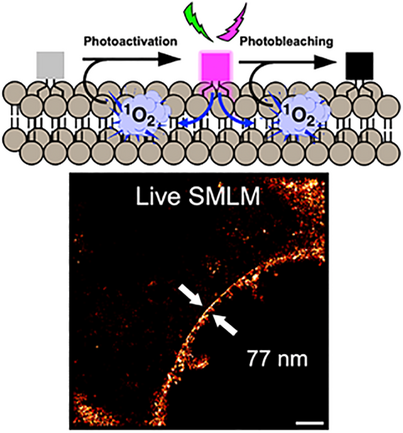
A Photoactivatable Plasma Membrane Probe Based on a Self-Triggered Photooxidation Cascade for Live Cell Super-Resolution Microscopy
Graphical Abstract
Herein, we establish a new photoswitching concept called self-triggered photooxidation cascade (STPC). Rhodamine dyes have mild photosensitizing properties that enable subsequent photoactivation and photobleaching of a leuco-rhodamine plasma membrane probe. Upon laser irradiation in the visible range, we successfully performed live SMLM (Single-molecule localization microscopy), allowing to image the plasma membrane at the nanoscale.
Abstract
Super-resolution imaging based on the localization of single emitters requires a spatio-temporal control of the ON and OFF states. To this end, photoactivatable fluorophores are adapted as they can be turned on upon light irradiation. Here, we present a concept called self-triggered photooxidation cascade (STPC) based on the photooxidation of a plasma membrane-targeted leuco-rhodamine (LRhod-PM), a non-fluorescent reduced form of a rhodamine probe. Upon visible light irradiation the small number of oxidized rhodamines, Rhod-PM, acts as a photosensitizer to generate singlet oxygen capable of oxidizing the OFF state LRhod-PM thereby switching it to its ON state. We showed that this phenomenon is kinetically favored by a high local concentration and propagates quickly when the probe is embedded in membrane bilayers. In addition, we showed that the close proximity of the dyes favors the photobleaching. At the single-molecule level, the concomitant activation/bleaching phenomena allow reaching a single-molecule blinking regime enabling single-molecule localization microscopy for super-resolution of live cellular membranes and their thin processes including filopodia and tuneling nanotubes.
Introduction
Photomodulation of fluorescent molecules draws particular attention due to their application in advanced bioimaging. Among them, photoactivatable probes are of particular interest in single-molecule localization microscopy to break the diffraction limit and reach super-resolution imaging.[1-7] Although photoactivatable fluorescent proteins have been developed for this endeavor,[8, 9] the latter remain limited to protein labeling and their brightness. Consequently, small fluorescent photoactivatable probes constitute an appealing alternative to proteins. In the last decades, several mechanisms have been proposed to develop photoactivatable fluorescent probes. Among other mechanisms,[10] two main different photoactivation processes can be distinguished. 1) Those that involve a photo elimination step, including nitrogen (N2) from a diazoketone[11] or azide-containing fluorophores,[3, 12] elimination of nitric oxide (NO),[13] photocleavage of spirooxazime,[6] and photo-uncaging of fluorescence quenchers.[14-17] 2) those involving an addition step, including protonation,[18, 19] and the addition of oxygen through a photooxidation process.[20-22] In this context, we recently established a new mechanism allowing us to obtain photoconvertible probes based on directed photooxidation,[23, 24] which enabled live single-molecule localization microscopy (SMLM) of mitochondria and plasma membrane (PM) on fragile samples like neurons.[25] Despite this last example, photoactivatable probes have rarely been targeted to the plasma membrane to perform live SMLM based on photoactivated localization microscopy (PALM). Indeed, there are only limited examples of SMLM performed at the plasma membrane using small fluorescent probes.[26] On fixed samples, several groups successfully resolved the PM.[27, 28] We notably reported that PM could be imaged at a nanoscopic scale on fixed neurons in two-color 3D-STORM using MemBright probes (MB-Cy3.5).[29] However, live SMLM on the cell surface is a challenging task due to the dynamic nature of the plasma membrane. Point accumulation for imaging in nanoscale topography (PAINT) approaches have successfully been applied to live SMLM of the PM, based on the transient binding of fluorogenic probes including solvatochromic Nile Red and self-quenched dimers with tuned lipophilicity.[30, 31] Additionally, BODIPYs showed interesting blinking properties based on PAINT,[32] or transient formation of red-shifted J-aggregates.[33] Finally, live SMLM of E. coli membrane has been performed using original rhodamine and silicon rhodamine.[34]
As referred above, rhodamine is an interesting scaffold to develop photoactivatable probes for live SMLM.[18, 20] Another potential way to trigger rhodamine fluorescence for PALM microscopy is to recover it from a non-emissive reduced leuco-rhodamine, also called dihydroxyrhodamine, that can be oxidized into its fluorescent rhodamine form. Indeed, dihydroxyxanthene scaffolds like DCHF (2′,7′-dichlorodihydrofluorescein) and DHR123 (dihydroxyrhodamine 123) are widely used to detect Reactive Oxygen Species (ROS).[35]
Interestingly, there are some concerns about the use of these ROS detectors, as they have been shown to provoke false positives while detecting ROS, due to their tendency to turn on upon “auto-oxidation” or photooxidation.[36, 37] However, by taking advantage of this photooxidation drawback, leuco-rhodamine dyes emerge as a promising scaffold to be used in PALM microscopy. The involvement of leuco-forms of cationic dyes like ATTO,[38] methylene blue,[39] has already been shown in dSTORM experiments on fixed samples.[40] Although the use of reduced fluorophore has already been shown to enhance single-molecule localization density for live super-resolution imaging, the use of exogenous, non-physiological sodium borohydride was required for super-resolution imaging.[41] Interestingly, Zhang et al. showed that dihydro silicon rhodamine could oxidize upon irradiation and used this feature for live SMLM in mitochondria.[42] In this work, we rationalized the use of leuco-rhodamine in live SMLM. We herein hypothesized that when targeted at the plasma membrane and upon irradiation, the small number of already oxidized rhodamines act as mild photosensitizers, able to oxidize their neighboring leuco-rhodamines in a concentration-dependent manner due to their homogeneous and free diffusion. This mechanism called self-triggered photooxidation cascade (STPC) results in a succession of photoactivation and photobleaching, which is a desirable feature for live SMLM.
Results and Discussion
Design & Synthesis
The design of our photoactivatable probe is based on several concepts. The first one relies on the fact that the leuco-Rhodamine (LRhod), the reduced form of a rhodamine is no longer fluorescent (OFF state) due to the disruption of the conjugation between the two phenyl rings of the xanthene moiety (Figure 1a). However, in oxidizing conditions, this LRhod can be reversibly oxidized into fluorescent rhodamine (ON state), Rhod. The second concept relies on the fact that, upon excitation, a fluorophore can generate singlet oxygen (1O2) through its excited triplet state. Consequently, we hypothesized that LRhod could get oxidized by a neighboring rhodamine, which acts as a photosensitizer (Figure 1a). To favor this mechanism, which relies on diffusion, we targeted LRhod to the plasma membrane. In the initial state, a small number of Rhod-PM molecules are already present on the cell surface (Figure 1b) due to ambient triplet oxygen, which is reactive enough to slowly oxidize leucorhodamines.[43] However, in the plasma membrane (PM) the probes freely diffuse, which favors the interaction between the photoactivatable probe LRhod-PM and the singlet oxygen generated by its oxidized cognate photosensitizer Rhod-PM. This interaction results in the photoactivation of LRhod-PM and since the 1O2 generated can be responsible for the photobleaching,[44] it can simultaneously lead to photobleaching of the emissive form (Figure 1c).
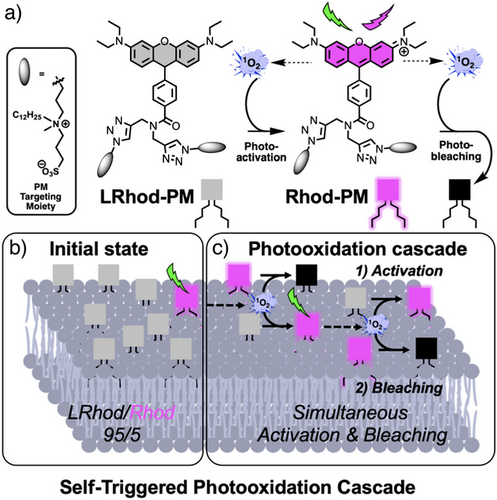
Overall, we thus expected that an efficient blinking system could be obtained in live SMLM imaging as the LRhod-PM probe alternatively turns ON and OFF, respectively, by photoactivation and photobleaching through an STPC mechanism.
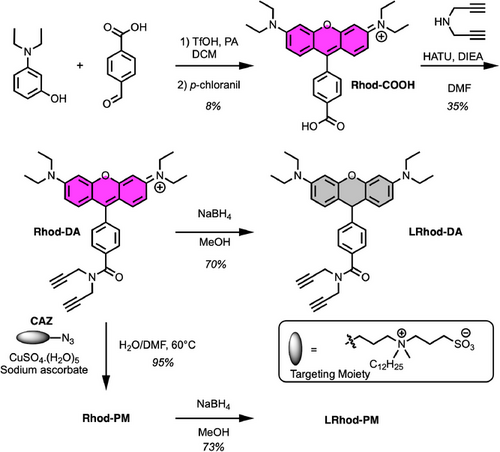
To obtain the PM probes, we first synthesized a functionalizable tetramethyl rhodamine Rhod-COOH possessing a carboxylate group (Scheme 1). The latter was conjugated to dipropargylamine to obtain Rhod-DA, which was either reduced by sodium borohydride to provide LRhod-DA or clicked to clickable amphiphilic zwitterion (CAZ) to get Rhod-PM, an approach giving rise to efficient fluorescent plasma membrane probes.[45, 29, 46-49] As a final step, Rhod-PM was reduced with sodium borohydride into the non-fluorescent plasma membrane probe leuco-rhodamine LRhod-PM.
Photophysical Properties
First, the photophysical properties of Rhod-PM and its precursors Rhod-DA and LRhod-DA were evaluated by spectroscopy and are reported in Figure 2. As expected, LRhod-DA did not absorb nor emit in the visible range (Figure 2a,b), while Rhod-DA displayed typical maximal excitation and emission wavelengths in methanol (560 and 585 nm, respectively) with a bright emission due to a high extinction coefficient (88200 M−1 cm−1) and a reasonable quantum yield of fluorescence (ϕF = 0.23).
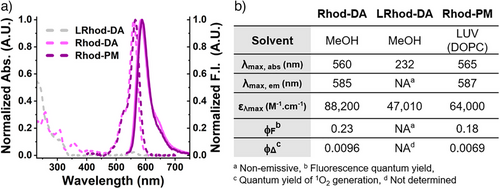
Rhod-PM rapidly binds to 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) large unilamellar vesicles (LUVs) (Figure S1) and, once embedded into this PM model, displayed slightly red-shifted spectra (Figure 2a) along with slightly reduced brightness compared to methanol (Figure 2b).
Self-Triggered Photooxidation Cascade in Bulk
To prove that a leuco-rhodamine can be photoactivated by a small amount of rhodamine following an STPC, we first assessed the ability of Rhod-DA to generate singlet oxygen as an oxidizing agent capable of oxidizing LRhod-DA. Since we expected the rhodamine to act as a photosensitizer, the quantum yields of singlet oxygen generation (ϕΔ) of Rhod-DA and Rhod-PM were evaluated through comparison with rose Bengal as a reference[50] and by monitoring the fluorescence decay of DPBF (Figure 3a). The results of 0.96% and 0.69% (Figure 2b) were in line with the low intersystem crossing quantum yields of rhodamines (0.5% to 1.0%),[51] and indicated that Rhod-PM could be used as a mild photosensitizer potentially able to oxidize LRhod-PM without being phototoxic to cells. Then, the ability of 1O2 to oxidize LRhod-DA was assessed by spectroscopy (Figure 3b). LRhod-DA was placed in a methanolic solution in the presence of 1O2 and the appearance of Rhod-DA was monitored by fluorescence spectroscopy. 1O2 was generated through the use of methylene blue, an efficient photosensitizer, which was independently excited (λEx = 638 nm, ϕΔ = 0.50)[50] and which provoked an increase in fluorescence intensity at 585 nm depicting the oxidation of LRhod-DA into its fluorescent oxidized form Rhod-DA. These experiments independently showed that Rhod-DA can be used as a photosensitizer to generate 1O2 and that the latter is able to oxidize LRhod-DA into its fluorescent Rhod-DA. Therefore, we assumed that Rhod-DA, by itself, could oxidize LRhod-DA. To check our assumption, a concentrated methanolic solution of LRhod-PM (10 µM) was irradiated at 532 nm by a laser beam over time. Upon irradiation, the fluorescence intensity integration increased for 2 h before slowly decreasing. The plotted curve corresponds to a typical autocatalysis sigmoid profile, relating to a first predominant photoactivation phase followed by a decrease assigned to a prevailing photobleaching phase (Figure 3c). This curve thus suggests 1) that Rhod-DA is generated over time and catalyzes the following oxidation of LRhod-DA and, 2) that upon its formation, Rhod-DA got photobleached. To verify our hypothesis, HPLC studies were conducted in parallel to monitor the photooxidation of LRhod-DA into Rhod-DA (Figure 3d). Before irradiation, both LRhod-DA (purple trace) and Rhod-DA (black trace) were analyzed by HPLC and revealed that LRhod-DA contained a small amount of the fluorescent Rhod-DA (≈5%, hashtag # in figure 3d, purple curve), probably due to oxidation over time by the oxygen contained in the air and solvents. Upon irradiation, the amount of LRhod-DA decreased (RT = 15.2 min), while Rhod-DA appeared (RT = 22.1 min) along with other photoproducts (e.g., RT = 21.2 min).
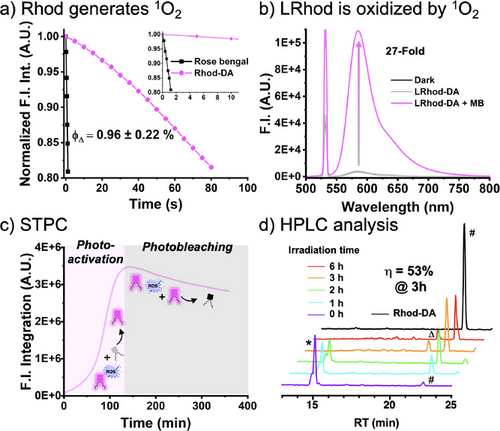
We also observed photoproducts that did not absorb in the visible range depicting photobleaching, whereas others were blue-shifted compared to Rhod-DA (peak indicated by Δ in figure 3d, λAbs max = 548 nm vs. 560 nm for Rhod-DA), corresponding to dealkylation upon irradiation.[52] After calibration (Figure S2), we were able to determine a maximum chemical yield of oxidation from LRhod-DA to Rhod-DA of 53% after 3 h of irradiation.
Overall, these analyses confirmed the STPC process where upon irradiation a small amount of rhodamine acts as a photosensitizer capable of oxidizing leuco-rhodamine through autocatalysis followed by its photobleaching.
Self-Triggered Photooxidation Cascade of LRhod-PM in PM Model
After demonstrating that LRhod could be used as a photoactivatable probe, we assessed the STPC of LRhod-PM at different local concentrations. When LRhod-PM was irradiated in methanol at 200 nM, i.e., at a low local concentration, virtually no fluorescence enhancement was observed, probably due to the fact that the small number of Rhod-PM present in the bulk was too diluted to trigger the photooxidation cascade of LRhod-PM (Figure 4a). Conversely, when LRhod-PM was incorporated into the PM model: DOPC large unilamellar vesicles (LUVs) at a probe/lipid ratio of 1/250, a fluorescence enhancement at 589 nm of 38-fold was observed in 20 min, and a quantum yield of photoactivation was determined, ϕPa = 5.8 × 10−5 (Figure 4b), which is in the range of photoactivatable fluorescent proteins.[53, 54] This phenomenon was assigned to the increase in the local concentration of probes, thus favoring the oxidation of LRhod-PM by its parent photosensitizer Rhod-PM.
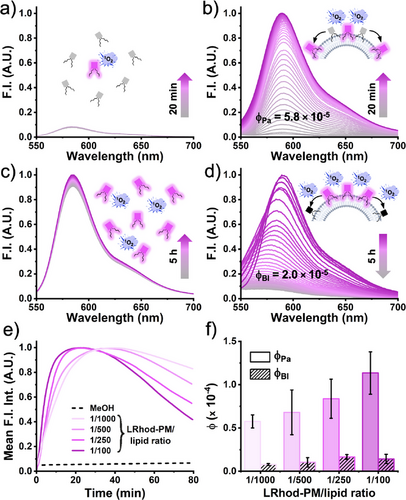
The same trend was observed for the photobleaching phenomenon when the fluorescent Rhod-PM was irradiated in methanol and in LUVs (Figure 4c,d). After 5 h of irradiation in methanol, a slight fluorescence increase was observed assigned to solvent evaporation while due to redistribution of the probe leading to a higher local concentration in LUVs, the kinetic of photobleaching was considerably increased compared to conditions in methanol when the probe homogeneously distributed, with a quantum yield of photobleaching, ϕBl of 2.0 × 10−5 (Figure 4d). It was noteworthy that upon photobleaching in the LUVs a significant blue shift was observed, which is in line with our previous HPLC analysis and the photo-dealkylation process.[52] Consequently, when localized in a lipid membrane, LRhod-PM alternatively undergoes enhanced photoactivation and enhanced photobleaching through an STPC due to increased local concentration. This hypothesis was further supported by the significant increase of both photoactivation and photobleaching kinetics upon the increase of local concentration through the variation of the probe/lipid ratio in the LUVs (1/100 to 1/1000) (Figure 4e). Indeed, the monitoring of the integrated signal over time combined with a kinetic model (see Supporting Information) allowed us to determine two kinetic constants kPa and kBl that were, respectively, assigned to the photoactivation and the photobleaching rates and yielded, respectively, the quantum yield of photoactivation ϕPa and of photobleaching ϕBl (Figure 4f). Interestingly, ϕPa was found to be 7 to 10-fold higher than ϕBl (Figure 4f). Overall, these experiments showed that Rhod-PM acts as a mild photosensitizer, able to oxidize LRhod-PM in an efficient manner when concentrated on a plasma membrane model through an STPC.
Photoactivation of LRhod-PM in Cell Imaging
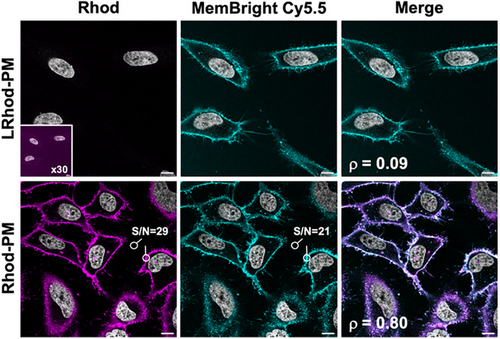
Once the proof of concept was established, we assessed the STPC mechanism on cells using LRhod-PM. As controls, the plasma membrane staining of the probes was checked in HeLa cells using laser scanning confocal microscopy. Although LRhod-PM did not produce any signal, Rhod-PM displayed a fast and wash-free fluorescent plasma membrane staining with a high signal-to-noise ratio of 29 and a Pearson's colocalization coefficient of 0.80 with MemBright-Cy5.5 (Figure 5).
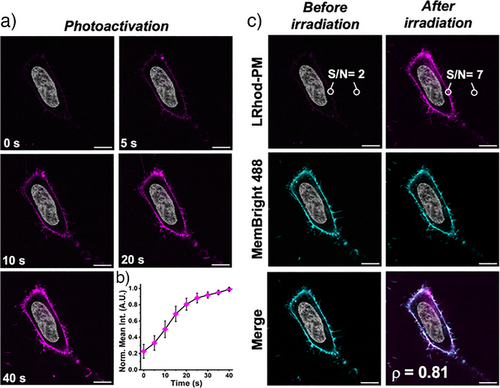
Then, cells incubated with LRhod-PM were irradiated for 40 s at 552 nm on a zoomed region at higher laser power (10%) and the cells were imaged over time (Figure 6a). Upon irradiation, the fluorescence signal progressively increased (5-fold) to reach a plateau after ≈30 s (Figure 6b, Supplementary movie 1). The obtained image displayed a good signal-to-noise ratio of 7 and indicated an efficient plasma membrane localization as proven by the high Pearson's colocalization coefficient with MemBright (Figure 6c).
Next, we confirmed that at the working concentration of 200 nM, both Rhod-PM and L-Rhod-PM were not cytotoxic nor photocytotoxic under irradiation using MTT cell viability assays in HeLa cells before and after irradiation (Figure S3). Overall, these cellular experiments successfully showed that L-Rhod can be used as an efficient photoactivatable plasma membrane probe without detrimental cytotoxic or photocytotoxic effects.
Self-Triggered Photooxidation Cascade Enables Live SMLM
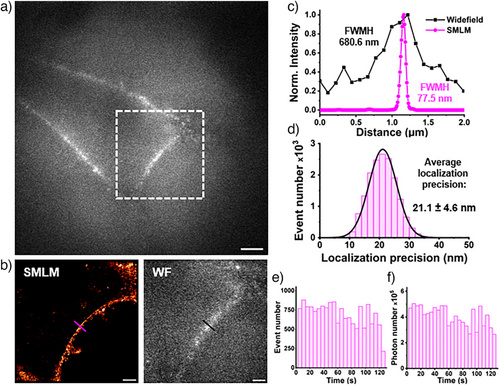
Common PALM probes typically require UV photoactivation,[10, 11, 14, 55] which is not preferable for live-cell applications. Conversely, LRhod-PM possesses an appealing feature for live-SMLM as it only requires a single laser in the visible range. Also, we assumed that the presented mechanism would find advantages in super-resolution imaging as it gathers the ideal conditions to perform live single-molecule localization microscopy (SMLM) in PALM conditions. Indeed, at the single-molecule level, an efficient photoactivation combined with an accelerated photobleaching would provide an efficient blinking profile and enable to reach a single-molecule regime where single emitters are temporally and spatially well separated.[56] However, in live super-resolution imaging based on SMLM acquisition time should be low, i.e., the number of blinks should be relatively high to have an efficient reconstruction and to palliate the potential movement of the cell during imaging. HeLa cells were then stained with LRhod-PM at a low concentration of 20 nM. In widefield microscopy, the low signal-to-noise ratio virtually did not enable the discrimination of the plasma membrane. Consequently, the cells and focal plane were found using nuclear staining in an independent channel (Hoechst, λEx: 405 nm). Upon irradiation of a region of interest (Figure 7a) at 532 nm (0.25 kW cm−2) a high frequency of blinking events was obtained and the single-molecule regime could be reached (See Supplementary movie 2). Importantly, the laser power density used here was significantly lower than many conventional SMLM experiments, typically 1 to 3 kW cm−2.[57] This blinking efficiency allowed the reconstruction of the plasma membrane from a 120 s acquisition only (9k frames at 14 ms frame rate) and with a high gain of resolution (Figure 7b). Indeed, while the widefield image after irradiation provided a membrane thickness of 681 nm, the SMLM image indicated 77 nm (Figure 7c). Due to the high brightness of Rhod-PM and its efficient excitation with the 532 nm laser line, the blinking event displayed a rather high mean localization precision of 21.1 ± 4.6 nm (Figure 7b). Moreover, the localized photooxidation of LRhod provided a fairly stable number of localizations along with a constant number of photons over time (Figure 7e,f).
In parallel, Rhod-PM was also evaluated for live SMLM. As expected, the same mean photon number per blink were obtained when using LRhod-PM and Rhod-PM since the former arises from the latter upon activation (Figure S4). Although reconstruction after acquisition provided an image of the plasma membrane (Figure S5), the use of Rhod-PM evidenced some drawbacks compared to LRhod-PM. Despite the use of poly-lysine-coated glass, Rhod-PM, as a cationic probe is prone to stick on the glass surface which induces a high background noise. This significantly reduces the localization density and the reconstructed image quality, which is depicted by the signal-to-noise ratio in widefield after irradiation (Figure S4B). Finally, while a cell stained with LRhod-PM reaches a single-molecule regime after only a few seconds, Rhod-PM, which is intrinsically fluorescent, needs to be photobleached for almost 1 min, which thus considerably reduces the reservoir of available blinking probes and increases the phototoxicity. This was illustrated by the mean event number of LRhod-PM which is twice higher than those of Rhod-PM over the acquisitions (Figure S4c, Supplementary movie 2). Overall, LRhod-PM enabled live SMLM imaging of plasma membrane in HeLa cells via STPC mechanism.
With LRhod-PM in hand, we then investigated the imaging of other PM protrusions and processes. Filopodia are finger-like projections that extend from the cell surface and play crucial roles in various cellular processes, including cell migration and signal sensing.[58] Similarly, tunneling nanotubes (TNTs) are thin, tube-like PM processes that form transiently between cells, enabling direct intercellular communication.[59] These PM structures are prone to movement and are thus challenging to image. However, using rapid acquisitions with LRhod-PM (1 min 24 and 28 s, respectively), we successfully imaged these thin processes in live HeLa cells with enhanced resolution (Figure 8a,b).
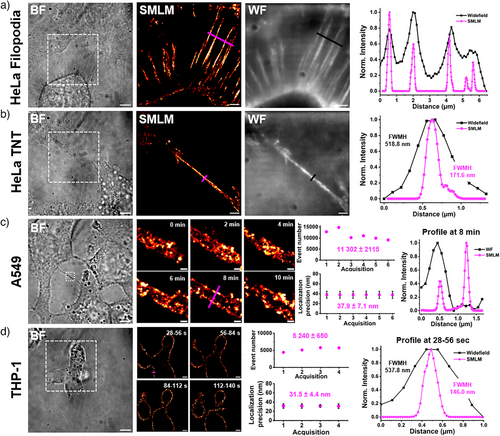
LRhod-PM was subsequently applied to two other cell lines to demonstrate its versatility: A549, human lung cancer epithelial cells (Figure 8c), and THP-1, human monocytes differentiated into macrophages (Figure 8d). Successive acquisitions were performed over 10 min on a TNT connecting two A549 cells. Owing to the performance of LRhod-PM, characterized by high blinking events over time and good localization precision despite the short acquisition time (14 ms), dynamic super-resolution imaging was achieved, resolving the two bilayers of the TNT (Figure 8c). Using the same approach, the movement and division of overlapping THP-1 cells were imaged at higher resolution, benefiting from the minimal fatigue associated with LRhod-PM's blinking performance (Figure 8d).
Conclusion
In conclusion, we herein synthesized LRhod-PM, a leuco-rhodamine plasma membrane-targeted probe, which can be photoactivated and excited with a single laser upon irradiation in the visible range and can be used in live SMLM (PALM) to rapidly obtain images of the membrane at the nanoscale. We first showed that the rhodamine Rhod-PM can act as a mild photosensitizer with a quantum yield of 1O2 generation lower than 1%. We then showed that 1O2 efficiently oxidizes the reduced form of rhodamine, called leuco-rhodamine. Then, we demonstrated that LRhod-PM could get photoactivated by Rhod-PM in a concentration-dependent manner. Similarly, the photobleaching of Rhod-PM is accelerated at high local concentrations when embedded in the lipid bilayer. This phenomenon, where a small number of Rhod-PM is sufficient to simultaneously trigger the photoactivation of L-Rhod and the photobleaching of Rhod-PM in an autocatalytic manner was called STPC. This mechanism, which was shown to be non-photocytotoxic to cells, was successfully applied to photoactivate the cell membrane in living cells as well as to perform live SMLM (PALM) imaging allowing to image the plasma membrane in 2 min with a high gain of resolution. In addition, owing to its high performance and low blinking fatigue, L-Rhod was employed to image thin PM processes (TNTs) and cell movements in various cell lines over time and at the nanoscale, thereby pushing the limits of the spatiotemporal resolution in cell imaging.
This work shows the application of leuco-rhodamines in live SMLM and provides a new PM probe adapted to the orange channel using a single common laser line (530–560 nm) with low laser power and without any cytotoxic UV photoactivation. We expect that this mechanism will pave the way to a new generation of targeted and multicolor fluorescent probes adapted to challenging super-resolution imaging of live samples.
Supporting Information
Protocols of synthesis and characterizations (1H NMR 13C NMR, HPLC-High-resolution mass spectrometry) of the synthesized probes can be found in Supporting Information as well as the method to determine kinetic constants, additional experiments, and supplementary figures.
Supplementary movie 1. Photoactivation of LRhod-PM.
Supplementary movie 2. SMLM acquisition Rhod-PM vs LRhod-PM.
Acknowledgements
This work was funded by the Agence Nationale de la Recherche: ANR 5D-SURE ANR-21-CE42-0015. The authors would like to thank Pr. Pascal Didier, Dr. Andrey S. Klymchenko, Dr. Alexandre Specht, Tania Steffan, Pr. Françoise Pons, Samuel Arca, Dr. Magalie Bénard and Dr. Ludovic Galas for their help.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.