Multivariate Distribution Structured Anisotropic Inorganic Polymer Composite Electrolyte for Long-Cycle and High-Energy All-Solid-State Lithium Metal Batteries
Graphical Abstract
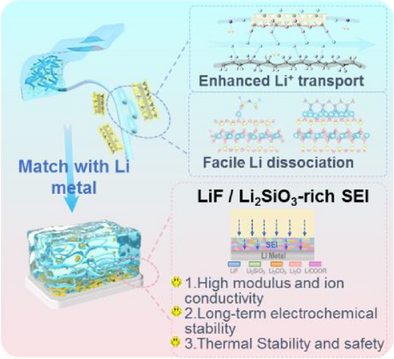
A composite solid-state electrolyte with multivariate distribution anisotropic structure was developed. Parts of the vermiculite sheets suspended among the PVDF matrix facilitate lithium salts dissociation and form fast Li+ transport channels. Other parts of vermiculite sheets tiled on electrolyte surface generate dense and high-modulus Li2SiO3-rich solid electrolyte interphase, improving the interfacial kinetics and suppressing the dendrite growth.
Abstract
Solid polymer electrolytes are promising candidates for solid-state Li metal batteries owing to their favorable rheological properties and interfacial compatibility with cathodes and Li anodes. However, their limited ionic conductivity and low modulus lead to inferior electrochemical performance and dendrite growth. Herein, we developed a composite solid-state electrolyte comprising vermiculite sheets and a poly(vinylidene fluoride) (PVDF) matrix with multivariate distribution and an anisotropic structure. Within this assembly, some vermiculite sheets were suspended in the PVDF matrix to facilitate Li salt dissociation and Li+ transport, while others were tiled on the electrolyte surface, generating a dense, high-modulus Li2SiO3-rich solid electrolyte interphase via in situ electrochemical reduction, which further improved interfacial kinetics and suppressed dendrite growth. As a result, a high conductivity of 1.38 mS cm−1 was achieved at room temperature, and the Li||Li cells displayed robust stability over 3000 h. The LiNi0.6Co0.2Mn0.2O2||Li full cells delivered a specific capacity of 172 mAh g−1 at 0.2 C and 86% capacity retention after 500 cycles at 0.5 C. Additionally, practical cycle performance at a high loading (4.4 mAh cm−2) was achieved in pouch cells. Overall, multivariate distribution and anisotropic structuring offers a novel perspective for the preparation of high-performance solid-state electrolytes.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.