A New Life For Nitrile-Butadiene Rubber: Co-Harnessing Metathesis And Condensation For Reincorporation Into Bio-Based Materials
Graphical Abstract
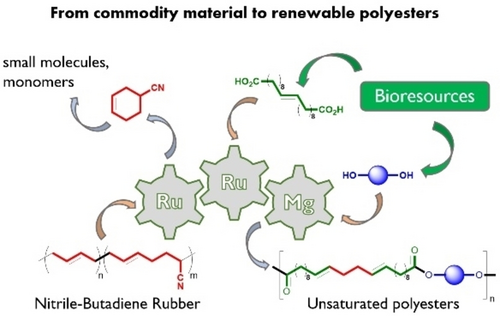
A key polymer material, nitrile-butadiene rubber (NBR), is converted under mild conditions via a one-pot catalytic sequence to bio-based polyesters with highly modulable structure and properties. The overall process operates with high atom-economy, formally redistributing the NBR monomers into new difunctional monomers as well as a single valuable small molecule by-product.
Abstract
Efficient plastic recycling processes are crucial for the production of value-added products or intermediates. Here, we present a multicatalytic route that allows the degradation of nitrile-butadiene rubber, cross-metathesis of the formed oligomers, and polymerization of the resulting dicarboxylic acids with bio-based diols, providing direct access to unsaturated polyesters. This one-pot approach combines the use of commercially available catalysts that are active and selective under mild conditions to synthesize renewable copolymers without the need to isolate intermediates.
Introduction
Due to their ubiquity and lack of circularity, polymers pose a serious environmental threat as plastic waste proliferates in landfills and aquatic environments. Most post-consumer plastics are currently downcycled for reuse,1-3 but global organizations and governments are seeking more effective solutions to address the environmental and economic impacts of the plastics crisis.4, 5 Therefore, the development of effective recycling processes within a circular economy approach is paramount, ideally producing not only monomers for new plastics, but also value-added products or intermediates for other supply chains.6, 7-15 Chemical recycling to monomers is an emerging method to reduce the accumulation of plastic waste, but it struggles to overcome the low cost of virgin monomers.16, 17 Upcycling of polymers is another promising approach that converts waste plastics/polymers into useful, value-added materials.18 Polymer upcycling schemes can also provide lower energy pathways and minimal environmental impact compared to traditional mechanical and chemical recycling.19 The main challenge is then to develop new methods to produce chemicals that are more valuable than monomers, polymers, or steam cracker feedstocks.20 In this regard, one-pot catalytic processes have significant advantages over multi-step syntheses, as they allow multiple synthetic transformations to be performed while avoiding purification steps.21 One-pot synthesis is therefore not only a valuable strategy to follow for the formation of new molecules, but also a promising green approach for the valorization of polymer waste.
Nitrile-butadiene rubber (NBR) is a copolymer composed of acrylonitrile (A) and butadiene (B). Although its physical and chemical properties vary depending on the exact composition, this synthetic rubber is highly resistant to oil, fuel and other chemicals: the more nitrile in the polymer, the higher the oil resistance, but the lower the flexibility of the material. As a result, NBR is most commonly used where high oil resistance is required, such as automotive seals, gaskets, or hoses for oil products. NBR is also used in textiles, where its application to woven and nonwoven fabrics improves the finish and waterproofing properties. The end of life of NBR products depends on the product itself. In general, NBR products are disposed of in a landfill, where they will eventually degrade and release chemicals into the environment. If the product is upcycled, it can be transformed into a more valuable compound, reducing landfill waste and environmental pollution while being added to a new value chain. In particular, the development of one-pot processes that allow the use of degradation products in the production of bio-based derivatives can contribute to the transition to a circular economy.22 Here, we present a practical route to bio-based unsaturated polyesters via a one-pot catalytic sequence using commercial catalysts and we demonstrate that these requirements can be achieved using the formation of butadiene oligomers as intermediates. This approach yields (partially) bio-based monomers and their corresponding copolymers in high yields.
Results and Discussion
Olefin metathesis is an extremely versatile tool for polymer chemists, largely due to the plethora of potential reaction schemes involving olefin-containing materials and substrates, as well as to the impressive robustness of current catalytic systems.23, 24 In addition, this versatility makes olefin metathesis a key reaction in an increasing number of multicatalytic reactions.25 For these reasons, olefin metathesis is an approach ideally suited for the development of upcycling processes, especially for diene-derived (co)polymers.26 In this view, we propose a modular approach for the upcycling of NBR, encompassing its consecutive metathetic conversion into difunctional, bio-enriched dicarboxylic acids and polycondensation with bio-based diols (Scheme 1). Interestingly, this allows full use of the comonomers from the starting rubber: part of the butadiene is incorporated within the polyester end products, while both butadiene and acrylonitrile combine into a valuable side-product.
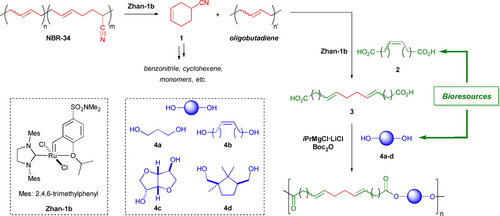
One-pot synthesis of unsaturated polyesters from NBR and bio-based diacids/diols.
For our studies, we selected a commercial batch of NBR containing 34 % of acrylonitrile, thus designated as NBR-34. 1H NMR confirms this butadiene/acrylonitrile ratio (Figure 1a). Detailed microstructure investigations were performed using 13C NMR spectroscopy (Supporting Information).27 The main structure consists of alternating A−B monomers, with short polybutadiene blocks. The material features a glass transition temperature at −19 °C. NBR has previously been subjected to olefin metathesis: after initial attempts with tungsten-based ill-defined systems,28 ruthenium-based second-generation Grubbs catalysts showed modest activity in converting this material.29 In view of its higher efficiency in cross-metathesis, we turned to the activated Hoveyda-Grubbs II catalyst, Zhan-1 b (Scheme 1). As recently pointed out, among the class of withdrawing group activated catalysts, Zhan-1 b remains an underexploited option, despite its potential.30 In addition to being more active, this catalyst has the advantage of being air stable and is claimed to be recyclable, which can reduce the cost of ruthenium catalysts in an upcycling process.31
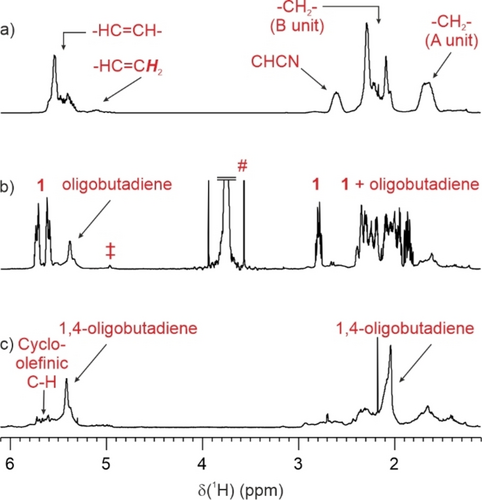
1H NMR spectra of a) NBR-34, b) crude reaction mixture after reaction in the presence of Zhan-1 b (0.19 % catalyst loading), 40 °C, 1 hour (#: dimethylcarbonate solvent, ≠: cyclododecatriene) and c) residual material after removal of volatiles.
The reaction conditions were optimized with two main objectives in mind: to evaluate milder reaction conditions and to use environmentally friendly solvents, as the reaction under neat conditions was not successful. Table 1 gathers the most salient data. Overall, satisfactory results are obtained in 1 h, under mild heating and with a modest catalytic loading of 0.19 % (calculated on the basis of 540 equivalents of NBR-34 double bond per Ru center). Longer reaction time or higher reaction temperature did not bring significant benefits in terms of process efficiency. Anisole, which allows the conversion of NBR-34 into smaller oligomers, appears to be the preferred reaction medium for the degradation of this copolymer.
Entry |
Temp (°C) |
Time (h)] |
Solvent |
1 (w %)[b] |
Res. PBD (w %)[c] |
|
Ð [d] |
|---|---|---|---|---|---|---|---|
1 |
40 |
1 |
DMC |
70.6 |
28.7 |
950 |
1.9 |
2 |
40 |
1 |
Acetone |
70.5 |
30.2 |
1250 |
1.8 |
3 |
40 |
4 |
Acetone |
66.7 |
33.3 |
1860 |
2.3 |
4 |
40 |
1 |
Anisole |
66.6 |
33.3 |
860 |
1.5 |
5 |
65 |
3 |
EtOAc |
65.0 |
35.0 |
1700 |
1.7 |
- [a] General conditions: 300 mg NBR-34 (3.67 mmol of B units), 5 mg of Zhan-1 b (6.81 μmol, 0.19 mol % catalyst loading), 4 mL solvent; [b] conversion of NBR into 1, defined as mass fraction of NBR converted into 1, determined by 1H NMR vs. internal standard; [c] weight ratio of residual, non-volatile material versus initial NBR-34; [d] determined by SEC in THF.
Figure 1b illustrates the conversion of NBR as monitored from in situ NMR. In the presence of a ruthenium catalyst under mild reaction conditions, an efficient conversion of the starting material was achieved within 1 hour: up to 70 w % was converted into 4-cyanocyclohexene (1). From a mechanistic point of view, this stems from the intramolecular ring-closing metathesis within a B−A−B sequence (Scheme 2). Interestingly, the formation of this compound in high yield was not reported in previous reports on the metathetical degradation of NBR. Under our reaction conditions, 1 is inert to any ring-opening metathesis reaction, due to low ring strain.32 This compound is of key importance in several applications. For example, its dehydrogenation over Pd catalysts readily affords benzonitrile,33 while iron-mediated extrusion of the cyano group has been reported to yield cyclohexene under mild conditions.34 This compound has also been used in polymer synthesis, as a precursor for polysulfones,35 or as a starting material for the preparation of diamino building blocks for polyamides and polyurethanes.36 For this purpose, compound 1 has always been formed by the Diels–Alder reaction between butadiene and acrylonitrile at high temperatures in low to moderate yields.37 In our case, it is prepared from NBR with high selectivity and under much milder reaction conditions.
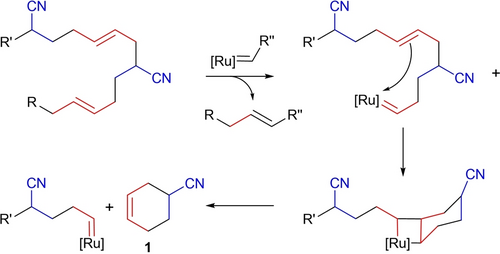
Formation of 4-cyanocyclohexene from a B−A−B sequence.
Having identified the optimal reaction conditions, we probe the scale-up of the NBR conversion, by converting a 3 g sample (Table S1). The outcome of the reaction was close to that observed for lower scale reactions, with slightly longer reaction time being required to reach similar mass average molar masses (Table S1, entry 2). Lowering the catalytic loading proved detrimental to the efficiency of the system (Table S1, entries 4 and 5). Even if further optimization would be required in the case of a process development, these results demonstrate that this first step is fully amenable for scale-up.
Examination of the non-volatile fraction revealed that the resulting material is predominantly 1,4-polybutadiene. Indeed, 1H and 13C NMR spectra feature characteristic signals at 5.40 and 129.1 ppm, respectively (Figure 1c and Supporting Information). In addition, unresolved multiplets at about 5.6 ppm are assigned to cyclopentene and cyclohexene groups within oligomers, that have been described as products from metathesis involving 1,2 butadiene fragments.38 Additional nitrile-related fragments are also present, though as minor components, based on IR intensity. As assessed by SEC, the size of the residual chains is within a 800 to 1600 g.mol−1 range, thus rather being considered as short chains or oligomers. Furthermore, DSC analysis indicates that the residual material features a glass transition temperature of −44 °C, 25 °C lower than that of pristine NBR.
With an efficient synthesis of unsaturated fragments in hand, we then sought to use our degradation products to prepare new unsaturated polyesters which are prevalent thermosetting polymers in the world.39 These materials are widely utilized as coatings or as constitutive matrices of glass fiber-based composites. Their good mechanical properties, low density, affordability, and ease of processing have made these formulations highly popular in a variety of industries including construction, electronics, and transportation.40 Furthermore, we envision new opportunities for these biobased thermoset resins by tailoring hydrophobicity, physical properties, and degradability of the polymer chains.41
In order to develop a simple methodology, we therefore decided to couple the oligomers obtained with a biobased diacid and subsequently form unsaturated polyesters by polycondensation (Scheme 1). Our efforts were focused on icos-10-ene-1,20-dioic acid (2) containing 16 CH2 units, which should increase the flexibility of the resulting polymer chain. This dicarboxylic acid is produced by the self-metathesis of 10-undecenoic acid, which itself result from the pyrolysis of ricinoleic acid, the main component of castor oil.42 Thus, the oligobutadienes derived from NBR self-metathesis were reacted with dioic acid 2, the telechelic cross-metathesis partner, resulting in formation of bis-unsaturated dioic acid 3, as evidenced by 1H and 13C NMR (Supporting Information). Furthermore, the =CH−CH2−CH2−CH= fragment formed features new, characteristic 1H and 13C NMR signals at 2.03 and 32.83 ppm, respectively. These give rise to a correlation in the 1H-13C HMBC heteronuclear NMR spectrum of 3, while showing proximity to an olefinic fragment as the sole neighbor (Supporting Information).
From these new compounds obtained by incorporating =CH−CH2−CH2−CH= fragments, we developed our one-pot sequence by step-growth polymerization of olefinic dicarboxylic acids with diols. As previously reported by our group, these polymerization reactions can occur under mild conditions using di-tert-butyl dicarbonate (Boc2O) as a coupling agent in the presence of a magnesium catalyst.43-46 In particular, magnesiate complexes, such as (TMP)MgCl ⋅ LiCl (TMP=2,2,6,6-tetramethylpiperidyl), which can easily deprotonate acids to carboxylates, making them more reactive for nucleophilic attack on dicarbonates, are efficient catalysts for the formation of polyesters.47, 48 We therefore investigated the efficiency of the related iPrMgCl ⋅ LiCl complex on the reaction between dioic acid 3 and a stoichiometric amount of propane diol.
The reaction was performed under mild reaction conditions (50 °C) in anisole, an environmentally benign solvent. In the presence of 0.6 equivalent of Boc2O, formation of the first coupling product, namely of the ester resulting from the dicarbonate-mediated condensation of 3 and 4 a, was evidenced by 1H NMR analysis of an aliquot collected after 23 h of reaction (Figure 2a). The spectrum features signals at 3.63 and 4.17 ppm in 1 : 1 ratio that account for the terminal CH2OH and CH2O(CO) protons, respectively. A faint signal assigned to intra-chain CH2O(CO) protons is observed at 4.08 ppm. Upon addition of further equivalents of Boc2O, the relative intensity of the intra-chain signal versus that of the terminal dipropanolate fragment evolved, as expected from chain growth (Figure 2b). In fine, sequential addition of 2.4 equivalents of dicarbonate results in a spectrum featuring a single OCH2 signal (Figure 2c). In parallel, monitoring by infrared spectroscopy of the reaction product shows gradual consumption of acid functionalities and formation of ester moieties, in line with the 1H NMR observations.

Copolymerization of 3 and 4 a: 1H NMR (left) and IR (right) follow-up of the reaction between 3 and 4 a in the presence of iPrMgCl.LiCl (4 mol % loading) in anisole at 50 °C, with sequential addition of a) 0.6 eq. Boc2O and 23 h reaction, b) 0.6 eq. Boc2O and 18 h reaction, and c) 1.2 eq. and 24 h reaction. *: residual signal from anisole.
Based on these preliminary investigations, to avoid the decomposition of the dicarbonate involved, we used sequential additions of Boc2O for the last step of our sequence. Under these conditions, the polyesterification of 2 with 4 a is efficiently achieved at 50 °C in the presence of iPrMgCl ⋅ LiCl to give a polymer with a Mw value of 6000 g.mol−1 and a dispersity of 1.5 (Table 2, Entry 1).
Entry |
Diol |
Time (h) |
[diol]/ [diacid] |
[Boc2O]/ [diacid] |
(g.mol−1) |
Ð [b] |
LogP/SA (Å−2)[c] |
|---|---|---|---|---|---|---|---|
1 |
4 a |
48 |
1.2 |
2.8 |
6160 |
1.5 |
0.0113 |
2 |
4 b |
48 |
1.1 |
2.2 |
15500 |
1.7 |
0.0118 |
3 |
4 c |
42 |
1.2 |
2.4 |
3480 |
1.9 |
0.0078 |
4 |
4 d |
94 |
1.1 |
2.8 |
4430 |
1.8 |
0.0128 |
- [a] General conditions: 3 prepared via one-pot procedure starting from NBR-34 (177 mg, 1.93 mmol of B units), 0.48 mmol diacid, 0.58 mmol diol, 19.3 μmol iPrMgCl LiCl, anisole (1 mL), 50 °C (See Supporting Information for more detailed description of reaction conditions), full conversion of diacid checked by 1H NMR of aliquots. [b] determined by SEC in THF. [c] octanol-water partition coefficients (LogP) normalized by surface area (SA) of polymer chain.
In addition to diol 4 a, further exploration of biosourced diols in Scheme 2 revealed that polyester hydrophobicity was comparable to NBR-34 (Figure S51). Furthermore, quantifying hydrophobicity of polymer chains with LogP/SA values indicated a more flexible biosourced alcohol, such as 4 b, was a great match for NBR-34 and much higher than many commercial aliphatic polyesters, like poly(butylene adipate).49
The synthetic strategy with diol 4 b, which is formally the reduction product of dioic acid 2, enabled us to obtain copolymers with higher molar masses up to 15500 g.mol−1 (Table 2, entry 2). It is well known that in order to maintain or improve the mechanical properties of biobased unsaturated polyesters, a balance between aliphatic and aromatic or cyclic compounds within the polyester backbone is necessary. Thus, we were able to quantitatively incorporate isosorbide (4 c) as a rigid comonomer, although with lower masses. Finally, for the copolymerization of 2 with a more sterically hindered alcohol, such as 4 d, iPrMgCl ⋅ LiCl converted both substrates in the presence of Boc2O, but with a longer reaction time (Table 2, entry 4).
The versatility of the one-pot synthesis approach was further exemplified with consecutive NBR-34 self-metathesis, followed by cross-metathesis with diol 4 b, and polycondensation with dicarboxylic acid 2 (Scheme 3). This afforded the expected copolymer (Mw=13 600 g/mol and Đ=2.4).
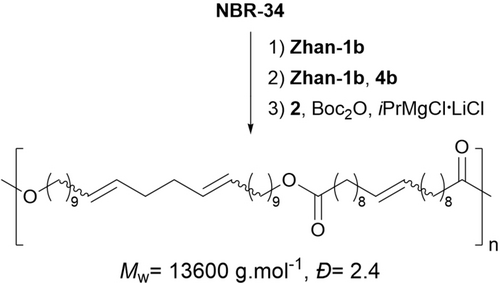
Polyester one-pot synthesis from NBR-34, diol 4 b and dioic acid 2.
Conclusion
Our multicatalytic sequence, which combines diene self-metathetic degradation, cross-metathesis and esterification, allows NBR to be upcycled into higher value unsaturated polyesters, minimizing the use of non-renewable resources and waste generation. This one-pot approach enables the synthesis of renewable copolymers without the need to isolate intermediates, and thus paves the way for the production of new, sustainable materials. Our future efforts will focus on investigating similar degradation processes and developing catalysts that can be recycled.
Supporting Information
The authors have cited additional references within the Supporting Information.50, 51
Acknowledgments
Chimie ParisTech – PSL, CNRS and Chaire Mines Urbaines are thanked for financial support. J.B. acknowledges the financial support of the El-yurt Umidi Foundation for his Ph.D. scholarship. Île-de-France Région is gratefully acknowledged for financial support of 500 MHz NMR spectrometer of Chimie ParisTech in the framework of the SESAME equipment project. We are grateful to Zongwei Zhang and Nargiz Kazimova for preliminary experiments. R.T.M. thanks Chimie ParisTech – PSL for a visiting professor fellowship.
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.



 [d]
[d] [b]
[b]
