Fundamentals of Unanticipated Efficiency of Gd2O3-based Catalysts in Oxidative Coupling of Methane
Graphical Abstract
Kinetic tests combined with temporal analysis of products revealed the fundamentals relevant for regulating the efficiency of the oxidative coupling of CH4 to C2H6 over Gd-based catalysts. When Gd2O3 is modified with Na, Sr or Ba, the use of N2O instead of O2 significantly improves the selectivity even at 650 °C. The promoters affect the binding strength of monoatomic oxygen species derived from N2O and thus control the methane oxidation to carbon oxides.
Abstract
Improving the selectivity in the oxidative coupling of methane to ethane/ethylene poses a significant challenge for commercialization. The required improvements are hampered by the uncertainties associated with the reaction mechanism due to its complexity. Herein, we report about 90 % selectivity to the target products at 11 % methane conversion over Gd2O3-based catalysts at 700 °C using N2O as the oxidant. Sophisticated kinetic studies have suggested the nature of adsorbed oxygen species and their binding strength as key parameters for undesired methane oxidation to carbon oxides. These descriptors can be controlled by a metal oxide promoter for Gd2O3.
Due to recent discoveries of natural/shale gas resources, the conversion of methane to ethane and ethylene (C2-hydrocarbons), oxidative coupling of methane (OCM), has gained new research interest.1 The use of O2 as an oxidant makes this reaction more thermodynamically feasible. However, C2-hydrocarbons are prone to oxidation to carbon oxides. Hindering these side reactions is a priority for OCM commercialization. The Mn/Na2WO4/SiO2 system, one of the promising OCM catalysts, shows reasonable selectivity above 800 °C.2 Catalysts based on oxides of lanthanides are also promising for OCM.3-6 La2O3,7 Sm2O38 or Nd2O39 can activate CH4 below 700 °C but have low selectivity to the desired products.
To control product selectivity, N2O, CO2, or S2 have been used instead of O2.10 N2O improved C2-hydrocarbons selectivity over various catalysts above 750 °C.11-13 Sm2O3 was the only rare earth oxide tested in OCM with N2O (N2O−OCM), but showed low selectivity.14 The use of N2O is advantageous from an environmental point of view, as the global warming potential of this compound is about 300 and 10 times higher than that of CO2 and CH4 respectively. One disadvantage of using N2O as oxidant is its price. N2O is, however, formed in large quantities as a by-product in the production of adipic acid,15 and can also be produced by the direct oxidation of NH3,16 which may become the basis for low-cost N2O production in the future. Motivated by these considerations, we introduce Gd2O3-based materials as a promising N2O−OCM system that is highly selective at 650–700 °C. Kinetic tests revealed how the overall pathways of the formation of C2-hydrocarbons and carbon oxides are affected by O2 and N2O. Temporal analyses of products enabled us to understand the role of promoter for Gd2O3 and type of oxygen species in the efficient conversion of CH4 to the target products.
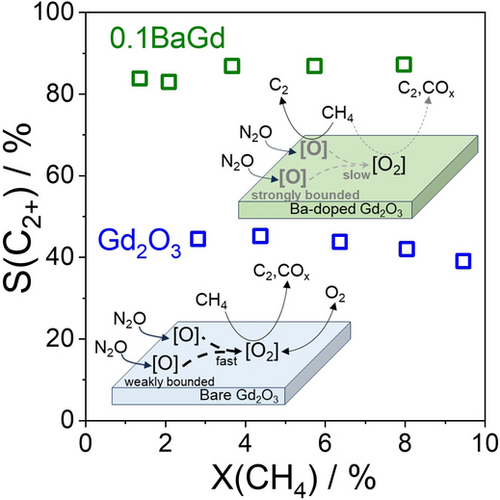
Oxides of Eu, Nd, Er, La, Gd, Ho, Dy or Sm were tested in O2-, and N2O−OCM at 750 °C (Figure 1). The selectivity to C2+-hydrocarbons (C2−C3 hydrocarbons) increased when O2 was replaced by N2O. The yield was almost unchanged over Sm2O3 and Dy2O3, decreased over Eu2O3 and Nd2O3 but increased over Ho2O3, La2O3, Er2O3 and Gd2O3 (to the highest extent). Gd2O3 was rarely used in OCM studies probably due to its low performance.6, 17 Ng and co-workers17 reported about 54 % selectivity to C2+-hydrocarbons at 32 % CH4 conversion over Gd2O3 modified with BaO in O2−OCM at 750 °C.
Results of OCM screening tests over (a) rare earth metal oxides or (b, c) Gd2O3-based catalysts with (a, b) O2 (blue symbols) and (a, c) N2O (green symbols). Reaction conditions: 40vol% CH4, CH4/O2=8, 750 °C, CH4/N2O=4, contact time τ is (a) 0.19 and (b, c) 0.047 gcat ⋅ min ⋅ mmolCH4−1.
Inspired by the positive effects of Na, Sr and Ba promoters on the C2-hydrocarbons selectivity of various catalysts,4 we modified Gd2O3 accordingly. The catalysts with 10 wt % promoter (0.1NaGd, 0.1SrGd and 0.1BaGd) consisted of the cubic Gd2O3 phase (Figure S1a). Ba and Sr were identified as carbonates. No Na-containing phase was detected. In situ and ex situ X-ray diffraction analysis of 0.1BaGd in O2−OCM and N2O−OCM revealed no change in phase composition (Figures S1b,c). Pseudo in situ X-ray photoelectron spectroscopy (XPS) tests also showed no obvious effect of the reaction feed on the catalyst surface composition. There are, however, some differences in the XP spectra of Gd2O3 and 0.1BaGd (Figures S1d–i). The XP C 1s spectrum of 01.BaGd is characterized by a signal at about 289 eV, which can be assigned to carbonates, probably barium carbonates in agreement with XRD data. The promoter should also be responsible for the presence of a signal at about 531 eV in the XP O1s spectrum.
The C2+-selectivity in O2−OCM over Gd2O3, 0.1NaGd, 0.1SrGd and 0.1BaGd at 650 °C is 33.6 %, 15.1 %, 30.8 %, and 22.6 %, respectively (Figure 1b, Table S1). When the temperature increased, the selectivity increased, too. Such a dependence is consistent with previous O2−OCM studies, which concluded that high temperatures are essential to achieve high C2+-selectivity.1, 2
Compared with O2−OCM, N2O−OCM over 0.1BaGd reached the selectivity of 77 % at 0.44 % CH4 conversion at 650 °C. The selectivity did not decrease even though the conversion increased to 7.5 % with increasing the reaction temperature to 800 °C (Figure 1c, Table S2). The selectivity of 0.1NaGd passed a maximum with increasing temperature while that of Gd2O3 and 0.1SrGd increased. Since the strongest positive effect was observed for 0.1BaGd, we further focused on this system. N2O−OCM tests with BaCO3 and BaO at 650 °C showed poor performance of these materials (Figure S2), so, Ba must be a promoter for Gd2O3. To understand the role of this promoter, BaGd catalysts with different Ba loading were further prepared and tested in O2−OCM and N2O−OCM.
In O2−OCM at 700 °C, the rate of overall CH4 consumption (r(CH4)) as well as the rates of CH4 conversion to C2H6 (r(C2H6)), C2H4 (r(C2H4)), CO2 (r(CO2)) and CO (r(CO)) decreased linearly with Ba loading (Figure 2a–c, Figure S3). Contrarily, an unexpected effect of Ba loading on these rates was observed in N2O−OCM. Although Ba addition reduced r(CH4) (Figure 2a), r(C2H6) followed a volcano-type dependence on the Ba/Gd ratio (Figure 2b). The r(C2H6) of all xBaGd was higher than that of Gd2O3. The r(C2)/r(COx) ratio of xBaGd in O2−OCM is close to 1, but is significantly higher in N2O−OCM, reaching its highest value of about 9 for 0.1BaGd (Figure 2d). Increasing the Ba loading further has, however, a negative effect. Importantly, r(CO2) and r(CO) decrease with Ba loading stronger in N2O−OCM than in O2−OCM (Figure S4).
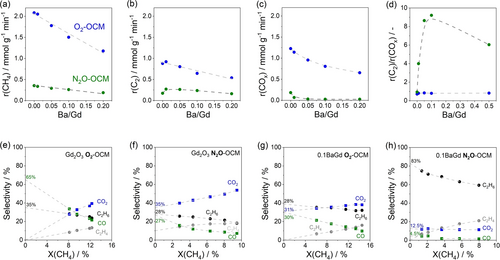
Effects of Ba loading on (a) r(CH4), (b) r(C2), and (c) r(COx). (d) The ratio of r(C2)/r(COx) versus Ba/Gd in O2- or N2O−OCM. (e–h) Primary selectivity in O2- or N2O−OCM over Gd2O3 and 0.1BaGd. Reaction conditions: 40vol% CH4, CH4/O2=8, CH4/N2O=4, 700 °C, τ is 0.037 gcat ⋅ min ⋅ mmolCH4−1 (O2−OCM) or 0.093 gcat ⋅ min ⋅ mmolCH4−1 (N2O−OCM).
The effects of O2 and N2O on the reaction pathways to CO, CO2, C2H4, and C2H6 were elucidated by analyzing the selectivity-conversion relationships obtained through varying the space velocity at 700 °C (Figure 2). To distinguish between primary (formed from CH4) and secondary (not formed from CH4) products, the selectivity to each product was extrapolated to zero CH4 conversion. The products with non-zero selectivity should be formed directly from CH4, while those with a zero value are secondary products derived from primary products. Regardless of the oxidant, CO and C2H6 are primarily products formed over 0.1BaGd or Gd2O3 (Figure 2e–h). This is also true for CO2 formation in N2O- and O2−OCM over 0.1BaGd and in N2O−OCM over Gd2O3.
Replacing O2 with N2O decreased the primary selectivity to C2H6 and CO over Gd2O3 from 35 to 28 % and from 65 to 27 %, respectively, but increased the primary selectivity to CO2 from 0 to 35 % (Figure 2e,f). Conversely, the primary selectivity to C2H6 over 0.1BaGd increased from 39 to 83 % (Figure 2g,h). This increase was accompanied by a decrease in the primary selectivity to CO and CO2 from 30 to 4.5 % and from 31 to 12.5 %, respectively. Thus, the modification of Gd2O3 with Ba is pivotal for improving the efficiency of CH4 conversion to C2H6 in the presence of N2O.
In both O2- and N2O−OCM, primarily formed C2H6 is converted to C2H4 as reflected by the decreasing and increasing dependence of the selectivity to these products on CH4 conversion (Figure 2). N2O appears to be superior to O2 in promoting this reaction as indicated by the higher selectivity ratio of C2H4 to C2H6 (Figure S5). The selectivity to CO and CO2 decreased and increased respectively with increasing methane conversion due to oxidation of CO to CO2. Since the selectivity to C2+-hydrocarbons or carbon oxides does not change significantly with increasing CH4 conversion, the desired products are not involved in consecutive oxidation reactions (Figure S6).
Direct N2O decomposition over Gd2O3 and 0.1BaGd was also investigated. Consistent with proposed mechanisms for this reaction over various catalysts,18 N2O first reacts with an anion vacancy ([ ]) to form gas-phase N2 and a monatomic adsorbed oxygen species ([O]) (eq 1. Gas-phase O2 can be formed by a reversible recombination of two [O] (eq 2 or an irreversible reaction of N2O with [O] (eq 3.
The dissimilar ability of Gd2O3 and its modified counterparts to form O2 from N2O was independently proved by analyzing the height-normalized responses of O2 and N2 (Figure 3a,b and Figure S8) recorded after pulsing of N2O:Ne=1 at 700 °C in a temporal analysis of products reactor.19 Since the O2 concentration is still high when the N2 concentration is zero, recombination of [O] originated from N2O should contribute to O2 formation.
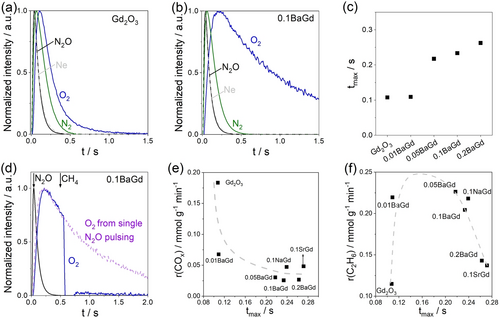
The height-normalized responses of N2O, N2 and O2 after pulsing N2O over (a) Gd2O3 and (b) 0.1BaGd. (c) The time of maximum intensity (tmax) of the height-normalized response of O2 after pulsing N2O over xBaGd. (d) The height-normalized responses of N2O and O2 after N2O−CH4 pump-probe tests with a time delay of 0.5 s over 0.1BaGd. (e) r(COx) in N2O−OCM versus tmax of O2. (f) r(C2H6) in N2O−OCM versus tmax of O2.
For a simple qualitative analysis of the rates of O2 and N2 formation, we use the time of maximum intensity (tmax) of the height-normalized O2 and N2 responses. For both unmodified and modified Gd2O3, O2 formation should limit N2O decomposition because tmax of O2 is significantly higher than tmax of N2 (Table S3). As the former value becomes larger in the presence of Ba promoter and with its rising concentration, the rate of [O] recombination should be hindered by the promoter probably due to an increase in the bonding strength of [O]. This statement is supported by the time required to reach zero O2 concentration (Figure S9).
The involvement of [O] in CH4 activation was verified by pump-probe experiments with N2O and CH4. When these gases were pulsed with a time delay (Δt) of 0.5 s, the tmax value of the O2 response did not change compared to that of single N2O pulses (Figures 3d and S10a). Its intensity, however, decreased sharply when CH4 entered the reactor because [O] oxidized this alkane to carbon oxides. C2H6 was not observed due to the high-vacuum conditions, which are unfavorable for recombination of methyl radicals. An increase in Δt caused a decrease in coverage by [O] due to recombination of these species to O2 (eq.2), which is detrimental to methane conversion (Figure S10b). The lower conversion of CH4 over Gd2O3 compared to 0.1BaGd is explained by the higher recombination rate of [O] to gas-phase O2. Lattice oxygen of Gd2O3 or BaO is significantly less active for CH4 oxidation than [O] as proven by pulse experiments with CH4 only (Figure S10c–f).
Based on the above results, we propose that the different activity of Gd2O3 and its modified counterparts to form O2 from N2O is the origin of the dissimilar behavior of these catalysts in O2- and N2O−OCM. Since the recombination of [O] to gas-phase O2 over the modified catalysts (eq 2) is slow, CH4 should react mainly with [O] in N2O−OCM. The much faster recombination of [O] over Gd2O3 favors the formation of gas-phase O2. Thus, O2−OCM could occur during N2O−OCM over Gd2O3. This scenario can explain why in contrast to Gd2O3 the selectivity to C2+-hydrocarbons is improved over xBaGd, 0.1NaGd and 0.1SrGd when O2 is replaced by N2O (Figures 1 and 2g,h). This explanation is indirectly supported by a negative relationship between tmax of the O2 response in N2O pulse tests and r(COx) or r(CH4) in N2O−OCM (Figures 3e, S11a). The stronger the oxygen species are bound, the lower their ability to oxidize CH4/C2-hydrocarbons to carbon oxides. The volcano-type relationship between r(C2H6) and Ba loading in Figure 2b can now be rationalized. This rate also follows a volcano-type relationship with tmax (Figure 3f). Importantly, 0.1NaGd and 0.1SrGd fit this relationship. Thus, an appropriate binding strength of [O] to the catalyst surface is vital to achieve the maximum r(C2H6), while the r(CH4) decreases with the strength due to the inhibition of COx formation (Figure S11b).
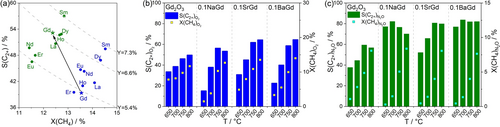
The impact of the performance of 0.1BaGd achieved in N2O−OCM is demonstrated by Figure 4a, where data from previous N2O−OCM studies with different catalysts are also presented (Table S4). The 0.1BaGd catalyst showed the selectivity to C2+ and C2-hydrocarbons of 89 % and 82 % at 3.5 % CH4 conversion, and 87 % and 80 % at 8 % CH4 conversion at 650 and 700 °C, respectively. Performing N2O−OCM with a feed containing 70 vol % CH4 further improved the selectivity to 89.8 % and 81 % at 11 % CH4 conversion. All catalysts tested so far were less efficient. Potassium lanthanide chlorides,16c Ca-actinide oxides,20 Ca-lanthanide oxides,6 Sm-based catalysts,16a LiMg-based catalysts,13 and Na2WO4-based catalysts13 operated at higher temperatures and achieved similar C2+-hydrocarbon yields but lower selectivity. According to a techno-economic analysis,21 however, the final price of ethylene is more influenced by selectivity than by methane conversion.
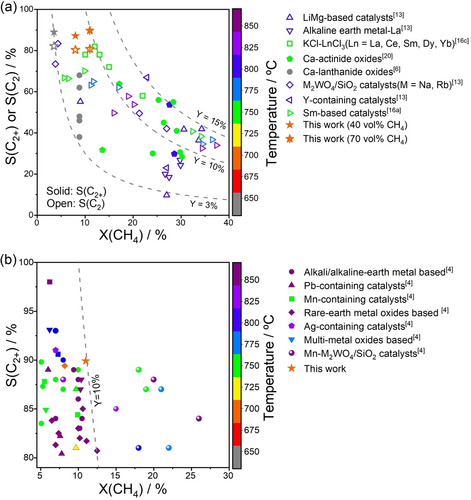
Selectivity-conversion values for C2- or C2+-hydrocarbons obtained in the present and previous (a) N2O−OCM or (b) O2−OCM studies. Current reaction conditions: τ is 0.75 or 0.43 gcat ⋅ min ⋅ mmolCH4−1, CH4/N2O=4. Literature data are given in Tables S4 for (a) and S5 for (b).
The C2+-selectivity of 90% at 11% CH4 conversion obtained in the present study in N2O−OCM at 700 °C is also remarkable in view of the O2−OCM data obtained over different catalysts summarized in the database of Ref.,4 which contains about 1800 selectivity-conversion data points. Using this database, we have selected catalysts showing the selectivity to C2-hydrocarbons above 80% at CH4 conversion degrees above 5% (Table S5). Only the most promising Mn/Na2WO4/SiO2 catalysts achieved the selectivity between 81 and 89 % at methane conversion above 15 % but above 750 °C (Figure 4b).
In conclusion, we have unveiled Gd2O3 modified with Ba, Na or Sr as a highly efficient system for N2O−OCM at 650–700 °C. The promoter enhances the binding strength of mono-atomically adsorbed oxygen species formed from N2O, which is pivotal for hindering their recombination to a diatomic adsorbed oxygen species, which shows higher ability for the direct CH4 oxidation to carbon oxides. The mono-atomic species appears to have a higher selectivity for the conversion of CH4 to C2H6. The established fundamentals provide clarity on the role of oxygen species in controlling product selectivity and can contribute to the design of OCM catalysts that also operate selectively with O2.
Acknowledgments
This work is supported by the National Natural Science Foundation of China (Grant Nos. 21961132026, 22225807, 22021004) and Deutsche Forschungsgemeinschaft (KO 2261/11-1) within a joint Sino-German research project. The authors thank Kathleen Schubert for ex situ XRD measurements. Kai Wu acknowledges the support from the China Scholarship Council. Open Access funding enabled and organized by Projekt DEAL.
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.