Hygroscopic Solutes Enable Non-van der Waals Electrolytes for Fire-Tolerant Dual-Air Batteries
Dr. Huarong Xia
Innovative Center for Flexible Devices (iFLEX), School of Materials Science and Engineering, Nanyang Technological University, 50 Nanyang Avenue, 639798 Singapore, Singapore
Search for more papers by this authorDr. Shengkai Cao
Institute of Materials Research and Engineering (IMRE), Agency for Science, Technology and Research (A*STAR), 2 Fusionopolis Way, 138634 Singapore, Singapore
Search for more papers by this authorDr. Zhisheng Lv
Institute of Materials Research and Engineering (IMRE), Agency for Science, Technology and Research (A*STAR), 2 Fusionopolis Way, 138634 Singapore, Singapore
Search for more papers by this authorDr. Jiaqi Wei
Innovative Center for Flexible Devices (iFLEX), School of Materials Science and Engineering, Nanyang Technological University, 50 Nanyang Avenue, 639798 Singapore, Singapore
Search for more papers by this authorSong Yuan
Innovative Center for Flexible Devices (iFLEX), School of Materials Science and Engineering, Nanyang Technological University, 50 Nanyang Avenue, 639798 Singapore, Singapore
Institute of Flexible Electronics Technology of THU, Tsinghua University, 314000 Jiaxing, Zhejiang, China
Search for more papers by this authorProf. Xue Feng
Center for Flexible Electronics Technology, Tsinghua University, No. 30, Shuangqing Road, 100084 Beijing, China
Search for more papers by this authorCorresponding Author
Prof. Xiaodong Chen
Innovative Center for Flexible Devices (iFLEX), School of Materials Science and Engineering, Nanyang Technological University, 50 Nanyang Avenue, 639798 Singapore, Singapore
Institute for Digital Analytics and Science (IDMxS), Nanyang Technological University, 50 Nanyang Avenue, 639798 Singapore, Singapore
Search for more papers by this authorDr. Huarong Xia
Innovative Center for Flexible Devices (iFLEX), School of Materials Science and Engineering, Nanyang Technological University, 50 Nanyang Avenue, 639798 Singapore, Singapore
Search for more papers by this authorDr. Shengkai Cao
Institute of Materials Research and Engineering (IMRE), Agency for Science, Technology and Research (A*STAR), 2 Fusionopolis Way, 138634 Singapore, Singapore
Search for more papers by this authorDr. Zhisheng Lv
Institute of Materials Research and Engineering (IMRE), Agency for Science, Technology and Research (A*STAR), 2 Fusionopolis Way, 138634 Singapore, Singapore
Search for more papers by this authorDr. Jiaqi Wei
Innovative Center for Flexible Devices (iFLEX), School of Materials Science and Engineering, Nanyang Technological University, 50 Nanyang Avenue, 639798 Singapore, Singapore
Search for more papers by this authorSong Yuan
Innovative Center for Flexible Devices (iFLEX), School of Materials Science and Engineering, Nanyang Technological University, 50 Nanyang Avenue, 639798 Singapore, Singapore
Institute of Flexible Electronics Technology of THU, Tsinghua University, 314000 Jiaxing, Zhejiang, China
Search for more papers by this authorProf. Xue Feng
Center for Flexible Electronics Technology, Tsinghua University, No. 30, Shuangqing Road, 100084 Beijing, China
Search for more papers by this authorCorresponding Author
Prof. Xiaodong Chen
Innovative Center for Flexible Devices (iFLEX), School of Materials Science and Engineering, Nanyang Technological University, 50 Nanyang Avenue, 639798 Singapore, Singapore
Institute for Digital Analytics and Science (IDMxS), Nanyang Technological University, 50 Nanyang Avenue, 639798 Singapore, Singapore
Search for more papers by this authorGraphical Abstract
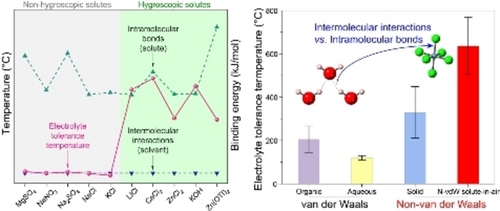
While the irreversible thermal transformation of electrolytes is typically attributed to solvent boiling, which disrupts solvent intermolecular interactions, our research revealed that hygroscopic solutes shift the determining factor to solute decomposition, breaking solute intramolecular bonds. As intramolecular bonds are much stronger, it enables ultrahigh thermal tolerance of non-van der Waals solute-in-air electrolytes.
Abstract
Thermal safety issues of batteries have hindered their large-scale applications. Nonflammable electrolytes improved safety but solvent evaporation above 100 °C limited thermal tolerance, lacking reliability. Herein, fire-tolerant metal-air batteries were realized by introducing solute-in-air electrolytes whose hygroscopic solutes could spontaneously reabsorb the evaporated water solvent. Using Zn/CaCl2-in-air/carbon batteries as a proof-of-concept, they failed upon burning at 631.8 °C but self-recovered then by reabsorbing water from the air at room temperature. Different from conventional aqueous electrolytes whose irreversible thermal transformation is determined by the boiling points of solvents, solute-in-air electrolytes make this transformation determined by the much higher decomposition temperature of solutes. It was found that stronger intramolecular bonds instead of intermolecular (van der Waals) interactions were strongly correlated to ultra-high tolerance temperatures of our solute-in-air electrolytes, inspiring a concept of non-van der Waals electrolytes. Our study would improve the understanding of the thermal properties of electrolytes, guide the design of solute-in-air electrolytes, and enhance battery safety.
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202318369-sup-0001-misc_information.pdf4 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aB. Dunn, H. Kamath, J. M. Tarascon, Science 2011, 334, 928–935;
- 1bP. G. Bruce, S. A. Freunberger, L. J. Hardwick, J.-M. Tarascon, Nat. Mater. 2012, 11, 19–29;
- 1cT. Liu, M. Leskes, W. Yu, J. Moore Amy, L. Zhou, M. Bayley Paul, G. Kim, P. Grey Clare, Science 2015, 350, 530–533;
- 1dL. Suo, O. Borodin, T. Gao, M. Olguin, J. Ho, X. Fan, C. Luo, C. Wang, K. Xu, Science 2015, 350, 938–943;
- 1eC. Xia, C. Y. Kwok, L. F. Nazar, Science 2018, 361, 777–781;
- 1fJ. M. Tarascon, M. Armand, Nature 2001, 414, 359–367;
- 1gH. Xia, Y. Tang, O. I. Malyi, Z. Zhu, Y. Zhang, W. Zhang, X. Ge, Y. Zeng, X. Chen, Adv. Mater. 2021, 33, 2004998;
- 1hZ. Zhu, Y. Tang, W. R. Leow, H. Xia, Z. Lv, J. Wei, X. Ge, S. Cao, Y. Zhang, W. Zhang, Angew. Chem. 2019, 131, 3559–3564;
- 1iZ. Zhu, Y. Tang, Z. Lv, J. Wei, Y. Zhang, R. Wang, W. Zhang, H. Xia, M. Ge, X. Chen, Angew. Chem. 2018, 130, 3718–3722;
- 1jH. Xia, W. Zhang, S. Cao, X. Chen, ACS Nano 2022, 16, 8525–8530.
- 2
- 2aK. Liu, Y. Liu, D. Lin, A. Pei, Y. Cui, Sci. Adv. 2018, 4, eaas9820;
- 2bL. Kong, C. Li, J. Jiang, M. G. Pecht, Energies 2018, 11, 2191.
- 3M.-T. F. Rodrigues, G. Babu, H. Gullapalli, K. Kalaga, F. N. Sayed, K. Kato, J. Joyner, P. M. Ajayan, Nat. Energy 2017, 2, 17108.
- 4
- 4aH. Yang, W. R. Leow, X. Chen, Adv. Mater. 2018, 30, 1704347;
- 4bL. Li, B. Fang, D. S. Ren, L. Fu, Y. Q. Zhou, C. Yang, F. S. Zhang, X. N. Feng, L. Wang, X. M. He, P. P. Qi, Y. Liu, C. Jia, S. Y. Zhao, F. Xu, X. D. Wei, H. Wu, ACS Nano 2022, 16, 10729–10741;
- 4cQ. Zhou, S. M. Dong, Z. L. Lv, G. J. Xu, L. Huang, Q. L. Wang, Z. L. Cui, G. L. Cui, Adv. Energy Mater. 2020, 10, 1903441;
- 4dX. N. Feng, D. S. Ren, X. M. He, M. G. Ouyang, Joule 2020, 4, 743–770;
- 4eZ. Chen, P. C. Hsu, J. Lopez, Y. Z. Li, J. W. F. To, N. Liu, C. Wang, S. C. Andrews, J. Liu, Y. Cui, Z. N. Bao, Nat. Energy 2016, 1, 15009.
- 5
- 5aX. L. Fan, J. Yue, F. D. Han, J. Chen, T. Deng, X. Q. Zhou, S. Hou, C. S. Wang, ACS Nano 2018, 12, 3360–3368;
- 5bH. F. Li, C. P. Han, Y. Huang, Y. Huang, M. S. Zhu, Z. X. Pei, Q. Xue, Z. F. Wang, Z. X. Liu, Z. J. Tang, Y. K. Wang, F. Y. Kang, B. H. Li, C. Y. Zhi, Energy Environ. Sci. 2018, 11, 941–951;
- 5cY. E. Hyung, D. R. Vissers, K. Amine, J. Power Sources 2003, 119, 383–387;
- 5dZ. Q. Zeng, B. B. Wu, L. F. Xiao, X. Y. Jiang, Y. Chen, X. P. Ai, H. X. Yang, Y. L. Cao, J. Power Sources 2015, 279, 6–12;
- 5eY. S. Ye, L. Y. Chou, Y. Y. Liu, H. S. Wang, H. K. Lee, W. X. Huang, J. Y. Wan, K. Liu, G. M. Zhou, Y. F. Yang, A. K. Yang, X. Xiao, X. Gao, D. T. Boyle, H. Chen, W. B. Zhang, S. C. Kim, Y. Cui, Nat. Energy 2020, 5, 786–793;
- 5fZ. Zeng, V. Murugesan, K. S. Han, X. Jiang, Y. Cao, L. Xiao, X. Ai, H. Yang, J.-G. Zhang, M. L. Sushko, J. Liu, Nat. Energy 2018, 3, 674–681;
- 5gF. Wang, O. Borodin, T. Gao, X. L. Fan, W. Sun, F. D. Han, A. Faraone, J. A. Dura, K. Xu, C. S. Wang, Nat. Mater. 2018, 17, 543;
- 5hW. Li, J. R. Dahn, D. S. Wainwright, Science 1994, 264, 1115–1118;
- 5iS. L. Liu, J. F. Mao, Q. Zhang, Z. J. Wang, W. K. Pang, L. Zhang, A. J. Du, O. R. Sencadas, W. C. Zhang, Z. P. Guo, Angew. Chem. Int. Ed. 2020, 59, 3638–3644;
- 5jS. J. Tan, J. Yue, X. C. Hu, Z. Z. Shen, W. P. Wang, J. Y. Li, T. T. Zuo, H. Duan, Y. Xiao, Y. X. Yin, R. Wen, Y. G. Guo, Angew. Chem. Int. Ed. 2019, 58, 7802–7807.
- 6X. N. Feng, M. G. Ouyang, X. Liu, L. G. Lu, Y. Xia, X. M. He, Energy Storage Mater. 2018, 10, 246–267.
- 7H. Xia, Z. Lv, W. Zhang, J. Wei, L. Liu, S. Cao, Z. Zhu, Y. Tang, X. Chen, Adv. Mater. 2022, 34, 2109857.
- 8M. Asadi, B. Sayahpour, P. Abbasi, A. T. Ngo, K. Karis, J. R. Jokisaari, C. Liu, B. Narayanan, M. Gerard, P. Yasaei, X. Hu, A. Mukherjee, K. C. Lau, R. S. Assary, F. Khalili-Araghi, R. F. Klie, L. A. Curtiss, A. Salehi-Khojin, Nature 2018, 555, 502–506.
- 9
- 9aJ. Lu, Y. Jung Lee, X. Luo, K. Chun Lau, M. Asadi, H.-H. Wang, S. Brombosz, J. Wen, D. Zhai, Z. Chen, D. J. Miller, Y. Sub Jeong, J.-B. Park, Z. Zak Fang, B. Kumar, A. Salehi-Khojin, Y.-K. Sun, L. A. Curtiss, K. Amine, Nature 2016, 529, 377–382;
- 9bH. J. Huang, D. S. Yu, F. Hu, S. C. Huang, J. N. Song, H. Y. Chen, L. L. Li, S. J. Peng, Angew. Chem. Int. Ed. 2022, 61, e202116068;
- 9cG. Li, X. L. Wang, J. Fu, J. D. Li, M. G. Park, Y. N. Zhang, G. Lui, Z. W. Chen, Angew. Chem. Int. Ed. 2016, 55, 4977–4982;
- 9dH. F. Wang, C. Tang, B. Wang, B. Q. Li, Q. Zhang, Adv. Mater. 2017, 29, 1702327.
- 10
- 10aL. Cao, D. Li, T. Pollard, T. Deng, B. Zhang, C. Yang, L. Chen, J. Vatamanu, E. Hu, M. J. Hourwitz, L. Ma, M. Ding, Q. Li, S. Hou, K. Gaskell, J. T. Fourkas, X.-Q. Yang, K. Xu, O. Borodin, C. Wang, Nat. Nanotechnol. 2021, 16, 902–910;
- 10bP. Yang, C. Feng, Y. Liu, T. Cheng, X. Yang, H. Liu, K. Liu, H. J. Fan, Adv. Energy Mater. 2020, 10, 2002898.
- 11Q. S. Nian, J. Y. Wang, S. Liu, T. J. Sun, S. B. Zheng, Y. Zhang, Z. L. Tao, J. Chen, Angew. Chem. Int. Ed. 2019, 58, 16994–16999.
- 12W. Sun, F. Wang, B. Zhang, M. Zhang, V. Küpers, X. Ji, C. Theile, P. Bieker, K. Xu, C. Wang, M. Winter, Science 2021, 371, 46–51.
- 13L. Wang, D. Snihirova, M. Deng, B. Vaghefinazari, W. Xu, D. Höche, S. V. Lamaka, M. L. Zheludkevich, Energy Storage Mater. 2022, 52, 573–597.
- 14
- 14aD. H. Wang, L. F. Wang, G. J. Liang, H. F. Li, Z. X. Liu, Z. J. Tang, J. B. Liang, C. Y. Zhi, ACS Nano 2019, 13, 10643–10652;
- 14bZ. W. Guo, Y. Y. Ma, X. L. Dong, J. H. Huang, Y. G. Wang, Y. Y. Xia, Angew. Chem. Int. Ed. 2018, 57, 11737–11741.
- 15
- 15aA. V. Shishkina, V. V. Zhurov, A. I. Stash, M. V. Vener, A. A. Pinkerton, V. G. Tsirelson, Cryst. Growth Des. 2013, 13, 816–828;
- 15bBrown, LeMay, Bursten, Murphy, Woodward, “Intermolecular forces”, can be found under https://chem.libretexts.org/@go/page/21770, 2022 (accessed: (2022, Sept. 17));
- 15cJ. Yang, R. Bai, B. Chen, Z. Suo, Adv. Funct. Mater. 2020, 30, 1901693.
- 16
- 16aX. Liu, Z. Wen, D. Wu, H. Wang, J. Yang, Q. Wang, J. Mater. Chem. A 2014, 2, 11569–11573;
- 16bT. Hibino, K. Kobayashi, M. Nagao, S. Kawasaki, Sci. Rep. 2015, 5, 7903;
- 16cX. B. Zang, R. J. Zhang, Z. Zhen, W. H. Lai, C. Yang, F. Y. Kang, H. W. Zhu, Nano Energy 2017, 40, 224–232;
- 16dZ. Pang, J. Duan, Y. Zhao, Q. Tang, B. He, L. Yu, J. Power Sources 2018, 400, 126–134;
- 16eD. W. Kim, S. M. Jung, H. Y. Jung, J. Mater. Chem. A 2020, 8, 532–542;
- 16fM. T. F. Rodrigues, K. Kalaga, H. Gullapalli, G. Babu, A. L. M. Reddy, P. M. Ajayan, Adv. Energy Mater. 2016, 6, 1600218;
- 16gP. Raut, W. F. Liang, Y. M. Chen, Y. Zhu, S. C. Jana, ACS Appl. Mater. Interfaces 2017, 9, 30933–30942;
- 16hM. Zhang, J. Zhang, J. Yang, J. Yao, Z. Chen, C. Lu, X. Du, Z. Zhang, H. Zhang, G. Cui, Chem. Commun. 2019, 55, 9785–9788;
- 16iR. R. Kohlmeyer, G. A. Horrocks, A. J. Blake, Z. N. Yu, B. Maruyama, H. Huang, M. F. Durstock, Nano Energy 2019, 64, 103927.
- 17P. Simon, Y. Gogotsi, B. Dunn, Science 2014, 343, 1210–1211.
- 18D. M. Pesko, M. A. Webb, Y. Jung, Q. Zheng, T. F. Miller, G. W. Coates, N. P. Balsara, Macromolecules 2016, 49, 5244–5255.
- 19W. Cao, J. Zhang, H. Li, Energy Storage Mater. 2020, 26, 46–55.
- 20The Engineering ToolBox, “Saturated Salt Solutions—Controlling Air Humidity”, can be found under https://www.engineeringtoolbox.com/salt-humidity-d_1887.html, 2014 (accessed: (2023, Dec. 20)).
- 21G. E. Walsberg, BioScience 2000, 50, 109–120.
- 22Merck, can be found under https://www.sigmaaldrich.com/SG/en, 2023 (accessed: (2023, Dec. 20)).
- 23The Engineering ToolBox, “Standard State and Enthalpy of Formation, Gibbs Free Energy of Formation, Entropy and Heat Capacity”, can be found under https://www.engineeringtoolbox.com/standard-state-enthalpy-formation-definition-value-Gibbs-free-energy-entropy-molar-heat-capacity-d_1978.html, 2017 (accessed: (2023, Dec. 20)).
- 24R. J. M. Konings, E. H. P. Cordfunke, W. Ouweltjes, J. Chem. Thermodyn. 1988, 20, 777–780.
- 25The Materials Project, can be found under https://next-gen.materialsproject.org/materials/mp-40886?chemsys=O−C−S−F-Zn, 2023 (accessed: (2023, Dec. 20)).
- 26C. W. Wu, X. Ren, W. X. Zhou, G. Xie, G. Zhang, APL Mater. 2022, 10, 040902.